Theralase(R) Demonstrates Effectiveness of X-Ray-Activated Drug
Rhea-AI Summary
Theralase (OTCQB: TLTFF) reported preclinical data (Nov 3, 2025) showing X-ray-activated Rutherrin enhances radiation therapy in multiple cancer models.
Key findings: 100-fold greater cancer cell kill vs radiation alone; >10-fold tumor uptake over healthy brain; complete tumor regression and durable protection in colorectal models; increased ROS, immune cytokine activation, and modulation of drug efflux pathways. The company plans GLP toxicology in 2026 to support clinical development targeting GBM, NSCLC, pancreatic, lymphoma and colorectal cancers.
Positive
- Cancer cell kill increased 100-fold
- Tumor uptake >10-fold vs healthy brain
- Complete tumor regression in colorectal models
- Durable protection on tumor rechallenge
- Increased ROS and immune cytokine activation
- Plans GLP toxicology for clinical entry in 2026
Negative
- Findings are preclinical only; no human data yet
- Clinical development depends on GLP toxicology in 2026
News Market Reaction
On the day this news was published, TLTFF declined 7.44%, reflecting a notable negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
Toronto, Ontario--(Newsfile Corp. - November 3, 2025) - Theralase® Technologies Inc. (TSXV: TLT) (OTCQB: TLTFF) ("Theralase®" or the "Company"), a clinical stage pharmaceutical company pioneering light, radiation, sound and drug-activated therapeutics for the treatment of cancer, bacteria and viruses, is pleased to share a scientific poster which demonstrates the effectiveness of X-Ray-activated Rutherrin® for numerous cancers in preclinical models.
Theralase® was unable to attend the presentation of the poster in person titled, "Rutherrin® Activated by Radiation Therapy Induces Synergistic Tumor Regression through Direct Destruction and Immune Activation in Multiple Preclinical Cancer Models."
The abstract has been published in the International Journal of Radiation Oncology - Biology - Physics at the following link:
The full poster is available at the following link:
Research Publications | Theralase Technologies Inc. - Theralase Technologies
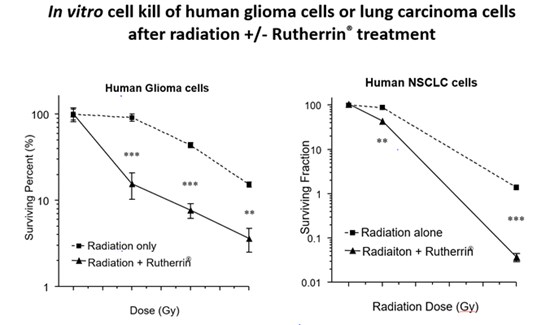
The study evaluated Theralase®'s lead drug formulation, Rutherrin® (a ruthenium-based small molecule formulated with recombinant human transferrin), activated by radiation therapy in a number of preclinical cancer models; including, glioblastoma multiforme (a deadly form of brain cancer), lung cancer (most common cancer), colorectal cancer (third most common) and lymphoma (seventh most common).
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/2786/272817_23f3f35354aa2a96_001full.jpg
Key Findings:
- Enhanced Tumor Destruction: Radiation-activated Rutherrin® achieved a 100-fold increase in cancer cell kill versus radiation therapy alone
- Oxidative and Immune Mechanisms: Rutherrin® increased Reactive Oxygen Species ("ROS") production and stimulated immune-related cytokine and chemokine expression, driving both immediate and immune-mediated cancer cell death
- Overcoming Resistance: The compound modulated drug efflux pathways, helping overcome mechanisms associated with multidrug and radiation resistance
- Durable Immune Memory: In vivo, Rutherrin® combined with radiation induced complete tumor regression in colorectal models and provided long-term protection against tumor rechallenge
- Tumor Selectivity and Survival: In GBM models, Rutherrin® demonstrated >10-fold preferential tumor uptake over healthy brain tissue and, in both GBM and lung models, significantly prolonged survival compared to radiation alone.
These key findings support Rutherrin® as a promising addition to radiation therapy, capable of improving local tumor control, overcoming treatment resistance and stimulating systemic immunity.
Mark Roufaiel, Ph.D., Research Scientist at Theralase® stated, "The preclinical research has demonstrated the utility of X-ray-activated Rutherrin® as a preferred addition to radiation therapy to increase efficacy, improve local tumor control, overcome treatment resistance and stimulate systemic immunity."
Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer of Theralase®, added, "Next steps in our research program are to complete Good Laboratory Practice toxicology studies in 2026, paving the way for the launch of clinical development targeting GBM, NSCLC, pancreatic, lymphoma and colorectal cancers."
Roger DuMoulin-White, B.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer of Theralase®, stated, "We are proud to share our scientific research demonstrating Rutherrin®'s unique characteristics in the destruction of cancer. The patented compound amplifies radiation's direct cytotoxic effects, while stimulating a durable immune response, positioning it as a potential breakthrough in the management of aggressive cancers."
About Theralase® Technologies Inc.
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and drug-activated small molecule compounds and their associated formulations with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses, with minimal impact on surrounding healthy tissue.
Additional information is available at https://theralase.com/ and www.sedarplus.ca.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-Looking Statements
This news release contains Forward-Looking Statements ("FLS") within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company's proposed development plans with respect to small molecules and their drug formulations. FLS may be identified by the use of the words "may, "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of the Company's management regarding future research, development and commercialization of the Company's small molecules; their drug formulations; preclinical research; clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete various clinical studies in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its small molecule and drug formulations; access to sufficient capital to fund the Company's operations is available on terms that are commercially favorable to the Company or at all; the Company's small molecule and formulations are effective against the diseases tested in its clinical studies; the Company's ability to comply with the terms of license agreements with third parties and as a result does not lose the right to use key intellectual property in its business; the Company's ability to protect its intellectual property; the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these FLS, which are not a guarantee of future performance. There can be no assurance that FLS will prove to be accurate as such FLS involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the FLS.
Although the FLS contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these FLS.
All FLS are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such FLS.
For investor information on the Company, please feel to reach out Investor Inquiries - Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
https://theralase.com/
Kristina Hachey, CPA
Chief Financial Officer X 224
khachey@theralase.com

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/272817







