Tevogen Highlights Potential Role of TVGN 489 in Eliminating Persistent Viral Reservoirs Linked to Long COVID
Tevogen Bio (NASDAQ:TVGN) has highlighted the potential of its investigational T cell therapy TVGN 489 in treating Long COVID, which affects an estimated 20 million Americans. Recent peer-reviewed studies have linked Long COVID to persistent viral reservoirs, where SARS-CoV-2 proteins and RNA remain in the body for months to years after infection.
TVGN 489, developed using Tevogen's ExacTcell™ platform, is an off-the-shelf cytotoxic CD8+ T lymphocyte therapy targeting multiple SARS-CoV-2 proteins. In proof-of-concept trials, the therapy demonstrated efficacy in reducing viral load in all patients, with CTLs persisting for at least 6 months without interfering with patients' immune responses.
The company is actively preparing for clinical manufacturing of TVGN 489, which could potentially restore homeostasis in Long COVID patients by eliminating virus-infected cells.
Tevogen Bio (NASDAQ:TVGN) ha evidenziato il potenziale della sua terapia con cellule T sperimentale TVGN 489 nel trattamento della Long COVID, che colpisce circa 20 milioni di americani. Studi peer‑review recenti hanno collegato la Long COVID a riserve virali persistenti, dove proteine e RNA di SARS‑CoV‑2 rimangono nel corpo per mesi o anni dopo l'infezione.
TVGN 489, sviluppata utilizzando la piattaforma ExacTcell™ di Tevogen, è una terapia off‑the‑shelf di linfociti T citotossici CD8+ mirata a multiple proteine di SARS‑CoV‑2. In trial di proof‑of‑concept, la terapia ha mostrato efficacia nel ridurre la carica virale in tutti i pazienti, con CTLs che persistevano per almeno 6 mesi senza interferire con le risposte immuni dei pazienti.
L'azienda si sta attivamente preparando alle lavorazioni cliniche di TVGN 489, che potrebbe potenzialmente ripristinare l'omeostasi nei pazienti Long COVID eliminando le cellule infette dal virus.
Tevogen Bio (NASDAQ:TVGN) ha destacado el potencial de su terapia de células T en investigación TVGN 489 para tratar Long COVID, que afecta a unos 20 millones de estadounidenses. Estudio revisados por pares recientes han vinculado el Long COVID con reservorios virales persistentes, donde proteínas y ARN de SARS‑CoV‑2 permanecen en el cuerpo durante meses o años después de la infección.
TVGN 489, desarrollado mediante la plataforma ExacTcell™ de Tevogen, es una terapia de células T citotóxicas CD8+ lista para usar que apunta a múltiples proteínas de SARS‑CoV‑2. En ensayos de concepto, la terapia demostró eficacia al reducir la carga viral en todos los pacientes, con CTLs presentes durante al menos 6 meses sin interferir con las respuestas inmunitarias de los pacientes.
La empresa se está preparando activamente para la fabricación clínica de TVGN 489, que podría restaurar la homeostasis en pacientes con Long COVID al eliminar células infectadas por el virus.
Tevogen Bio (NASDAQ:TVGN) 은 연구 중인 T 세포 치료제 TVGN 489 를 Long COVID 치료에 잠재력이 있다고 강조했습니다. Long COVID 는 약 2천만 명의 미국인 에 영향을 미칩니다. 최근 동료 평가를 거친 연구들은 Long COVID 가 바이러스 저장소의 지속으로 연결되며, SARS‑CoV‑2 단백질과 RNA 가 감염 후 수개월에서 수년 동안 체내에 남아 있음을 보여주었습니다.
TVGN 489 는 Tevogen 의 ExacTcell™ 플랫폼 을 이용해 개발된 즉시 사용 가능한 CD8+ 사이토톡식 T 림프구 치료제로, 다수의 SARS‑CoV‑2 단백질을 표적으로 합니다. 개념 검증 시험에서 이 치료는 모든 환자에서 바이러스 부하 감소를 보였고, CTLs 는 최소 6개월 동안 지속되었으며 환자의 면역 반응에 영향을 주지 않았습니다.
회사는 Long COVID 환자의 바이러스에 감염된 세포를 제거해 항상성을 회복할 potential 이 있는 TVGN 489 의 임상 제조를 적극적으로 준비 중입니다.
Tevogen Bio (NASDAQ:TVGN) a mis en évidence le potentiel de sa thérapie expérimentale par cellules T TVGN 489 dans le traitement du long COVID, qui touche environ 20 millions d'Américains. Des études évaluées par des pairs récentes ont relié le long COVID à des réservoirs viraux persistants, où les protéines et l'ARN du SARS‑CoV‑2 demeurent dans le corps pendant des mois voire des années après l'infection.
TVGN 489, développée grâce à la plateforme ExacTcell™ de Tevogen, est une thérapie prête à l'emploi à base de lymphocytes T CD8+ cytotoxiques visant plusieurs protéines du SARS‑CoV‑2. Dans des essais conceptuels, la thérapie a démontré une efficacité en réduisant la charge virale chez tous les patients, les CTLs persistant pendant au moins 6 mois sans perturber les réponses immunitaires des patients.
L'entreprise se prépare activement à la fabrication clinique de TVGN 489, qui pourrait potentiellement rétablir l'homéostasie chez les patients atteints de long COVID en éliminant les cellules infectées par le virus.
Tevogen Bio (NASDAQ:TVGN) hat das Potenzial seiner experimentellen T‑Zell-Therapie TVGN 489 zur Behandlung von Long COVID hervorgehoben, das nach Schätzung 2 Millionen Amerikaner betrifft. Jüngste Peer‑Review‑Studien haben Long COVID mit persistierenden Virusreservoiren in Verbindung gebracht, in denen SARS‑CoV‑2 Proteine und RNA Monate bis Jahre nach der Infektion im Körper verbleiben.
TVGN 489, entwickelt mit der Tevogen‑ExacTcell™‑Plattform, ist eine einsatzbereite zytotoxische CD8+-T‑Lymphozyten‑Therapie, die auf mehrere SARS‑CoV‑2‑Proteine abzielt. In Proof‑of‑Concept‑Studien zeigte die Therapie Wirksamkeit bei der Reduzierung der Viruslast bei allen Patienten, wobei CTLs mindestens 6 Monate lang nachweisbar waren, ohne die Immunantworten der Patienten zu beeinträchtigen.
Das Unternehmen bereitet sich aktiv auf die klinische Herstellung von TVGN 489 vor, die potenziell die Homöostase bei Long COVID‑Patienten wiederherstellen könnte, indem virusinfizierte Zellen eliminiert werden.
Tevogen Bio (NASDAQ:TVGN) أبرزت إمكانات علاجها التجريبي بالخلايا التائية TVGN 489 في معالجة مرض «كوفيد الطويل» Long COVID، الذي يؤثر على نحو 20 مليون أمريكي. ربطت دراسات مُراجَعة من قِبَل النظراء بين Long COVID ووجود مستودعات فيروسية مستمرة، حيث تبقى بروتينات وRNA لـ SARS‑CoV‑2 في الجسم لعدة أشهر إلى سنوات بعد الإصابة.
TVGN 489، المطوَّر باستخدام منصة Tevogen ExacTcell™، هو علاج خلايا T سيتوتوكسية من نوع CD8+ جاهز للاستخدام يستهدف عدة بروتينات لـ SARS‑CoV‑2. في تجارب المفاهيم الأولى، أظهر العلاج فاعلية في خفض الحمل الفيروسي لدى جميع المرضى، مع استمرار CTLs لمدة لا تقل عن 6 أشهر دون التأثير على استجابات المناعة لدى المرضى.
تعمل الشركة حاليًا على التحضير للتصنيع السريري لـ TVGN 489، الذي قد يعيد التوازن إلى مرضى Long COVID من خلال القضاء على الخلايا المصابة بالفيروس.
Tevogen Bio(纳斯达克股票代码:TVGN) 强调了其研究中的 T 细胞治疗 TVGN 489 在治疗 Long COVID 方面的潜力——该病估计影响约 2000 万名美国人。最近的同行评议研究将 Long COVID 与持续存在的病毒储库联系起来,在感染后数月到数年内,SARS‑CoV‑2 的蛋白质和 RNA 仍留在体内。
TVGN 489 使用 Tevogen 的 ExacTcell™ 平台 开发,是一种现成的 CD8+ 细胞毒性 T 淋巴细胞治疗,靶向多种 SARS‑CoV‑2 蛋白。概念验证试验显示,该治疗在所有患者中 降低病毒载量,CTLs 至少持续 6 个月且不干扰患者的免疫反应。
公司正积极为 TVGN 489 的临床制造做准备,这可能通过消除被病毒感染的细胞来帮助 Long COVID 患者恢复稳态。
- Proof-of-concept trial showed TVGN 489 reduced viral load in all patients
- CTLs persisted for 6 months in all tested patients without immune interference
- Therapy targets multiple SARS-CoV-2 proteins, not just Spike protein
- Addresses large market opportunity with 20 million affected Americans
- Additional research still needed to confirm efficacy
- Clinical manufacturing phase not yet initiated
- Will require additional capital to execute business plan
Insights
Tevogen highlights promising TVGN 489 T cell therapy that targets persistent SARS-CoV-2 viral reservoirs potentially causing Long COVID symptoms.
Tevogen is advancing an innovative approach to Long COVID treatment with their investigational precision T cell therapy, TVGN 489. The company's announcement connects several important scientific developments: First, recent peer-reviewed studies have confirmed that SARS-CoV-2 viral fragments can persist in the body for months or years after acute infection, creating viral reservoirs that likely trigger the chronic inflammation underlying Long COVID symptoms.
What makes TVGN 489 particularly noteworthy is its mechanism of action. Unlike treatments targeting only the Spike protein, this therapy deploys cytotoxic CD8+ T lymphocytes designed to recognize multiple SARS-CoV-2 proteins across the entire viral genome. This broad-spectrum approach addresses a crucial limitation in current COVID therapies, which may miss viral fragments from non-Spike proteins that contribute to Long COVID.
The preliminary clinical data looks promising: in a proof-of-concept trial, TVGN 489 reduced viral load in all patients, with the therapeutic T cells persisting for at least 6 months without interfering with patients' own immune responses. This persistence is particularly important for addressing a chronic condition like Long COVID.
The company is now preparing for clinical manufacturing, indicating they're advancing toward larger trials. With an estimated 20 million Americans suffering from Long COVID and limited treatment options available, Tevogen's approach represents a potentially significant advancement in addressing this substantial unmet medical need.
Tevogen's announcement positions TVGN 489 as a potential breakthrough in the Long COVID treatment landscape, where effective therapies are lacking despite affecting
Two critical aspects differentiate Tevogen's approach: First, their ExacTcell™ platform produces off-the-shelf allogeneic T cells, avoiding the logistical complexities and expenses of autologous cell therapies that require individual patient cell harvesting. Second, by targeting multiple viral proteins beyond just Spike, TVGN 489 may address viral fragments that escape current therapeutic approaches.
The preliminary clinical results provide encouraging proof-of-concept, but investors should note this is still early-stage. The company is only now preparing for clinical manufacturing, suggesting significant development work remains before potential commercialization. The press release doesn't mention timeline details for advanced trials or regulatory submissions.
Market opportunity is substantial if Tevogen succeeds—with 20 million Americans affected by Long COVID and limited treatment options, a therapy demonstrating clear efficacy could capture significant market share. However, challenges remain in definitively proving efficacy in a condition with diverse symptoms and biological mechanisms.
The announcement represents a positive development in Tevogen's pipeline but falls within expected R&D progression rather than indicating an immediate breakthrough. The company appears to be methodically advancing its asset while emphasizing its scientific rationale.
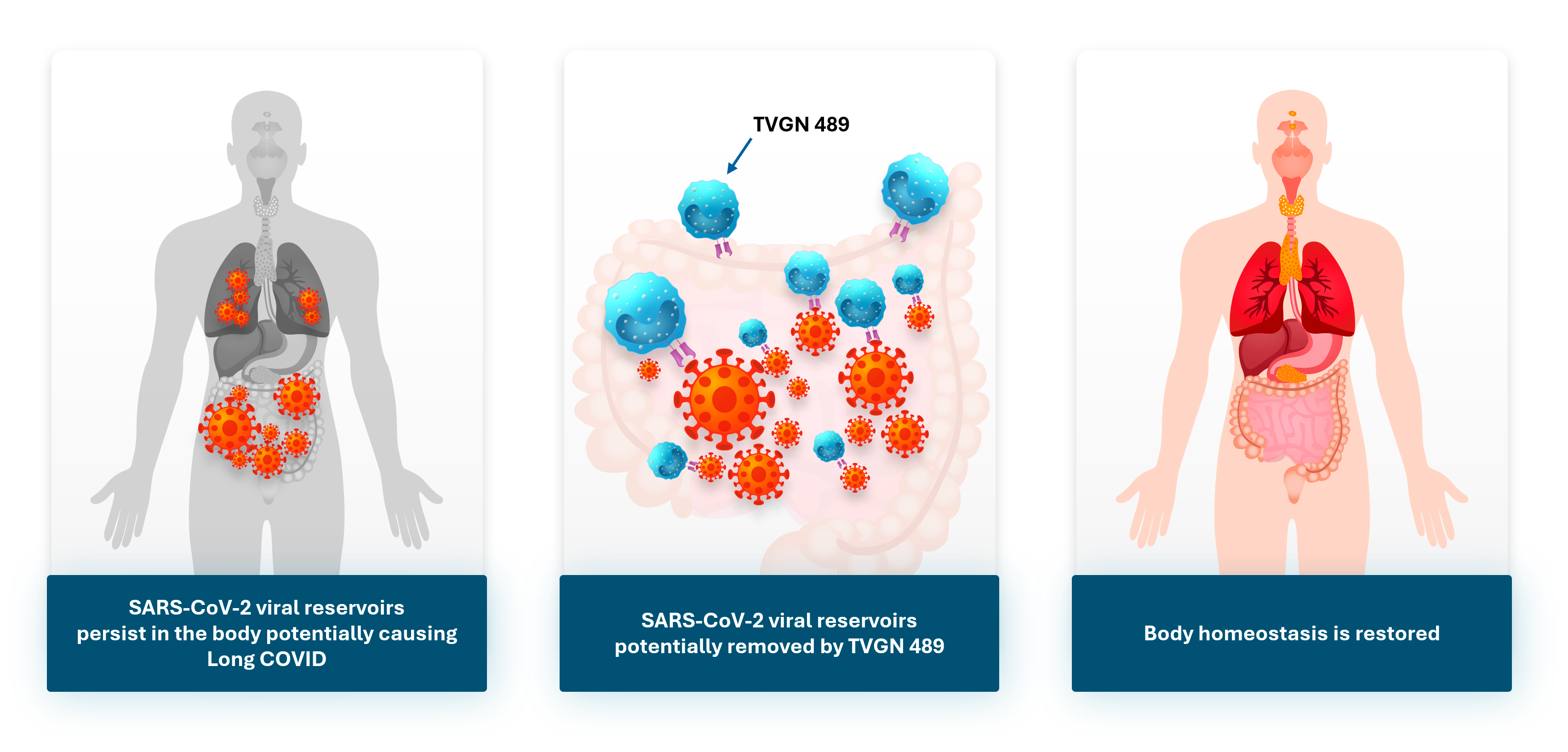
WARREN, N.J., Sept. 23, 2025 (GLOBE NEWSWIRE) -- Tevogen (“Tevogen Bio Holdings Inc.” or “Company”) (Nasdaq: TVGN), today highlights emerging scientific evidence linking persistent viral reservoirs to Long COVID, and emphasizes the potential of its investigational precision T cell therapy, TVGN 489, for the treatment of this debilitating condition which affects an estimated 20 million Americans and represents an area of unmet need.
Peer-reviewed studies such as the ones published in Sci Trans Med, Clin Microbial Infect and The Lancet Infectious Diseases report the detection of residual SARS-CoV-2 proteins and RNA for months to years after acute infection in individuals. This persistence suggests the presence of a viral reservoir which results in chronic immune inflammation and dysfunction underlying Long COVID symptoms. These findings open new avenues for therapeutic approaches aimed at eliminating the residual virus and restoring immune and physiological homeostasis.
Tevogen Bio’s first clinical product, TVGN 489, from its allogeneic precision T cell therapy platform, ExacTcell™, is an off-the-shelf cytotoxic CD8+ T lymphocyte (CTL) therapy designed to target multiple SARS-CoV-2 proteins across the entire viral genome, not just the Spike protein. In Tevogen Bio’s proof-of-concept clinical trial, TVGN 489 showed efficacy in reducing the viral load for all patients and TVGN 489 CTLs persisted for at least 6 months in all patients tested; the CTLs did not interfere with patient’s own immune responses. The Company believes that TVGN 489 may provide broad and durable immune responses against persisting SARS-CoV-2 fragments, including those originating from proteins other than Spike (Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae | Clinical Infectious Diseases | Oxford Academic), potentially representing a strategy for Long COVID treatment.
“These recent scientific reports strengthen the theory that Long COVID is, at least in part, sustained by a persistent viral reservoir,” said Dr. Ryan Saadi, Founder and CEO of Tevogen. “Our investigational therapy, TVGN 489, is uniquely designed to eliminate virus-infected cells. Tevogen is actively preparing for clinical manufacturing of TVGN 489 and while more research is needed, based on positive dose-finding trial data, I am highly optimistic that TVGN 489 has the potential to restore homeostasis in patients suffering from this debilitating condition.”
Forward Looking Statements
This press release contains certain forward-looking statements, including without limitation statements relating to: Tevogen’s plans for its research and manufacturing capabilities; expectations regarding future growth; expectations regarding the healthcare and biopharmaceutical industries; and Tevogen’s development of, the potential benefits of, and patient access to its product candidates for the treatment of infectious diseases and cancer. Forward-looking statements can sometimes be identified by words such as “may,” “could,” “would,” “expect,” “anticipate,” “possible,” “potential,” “goal,” “opportunity,” “project,” “believe,” “future,” and similar words and expressions or their opposites. These statements are based on management’s expectations, assumptions, estimates, projections and beliefs as of the date of this press release and are subject to a number of factors that involve known and unknown risks, delays, uncertainties and other factors not under the company’s control that may cause actual results, performance or achievements of the company to be materially different from the results, performance or other expectations expressed or implied by these forward-looking statements.
Factors that could cause actual results, performance, or achievements to differ from those expressed or implied by forward-looking statements include, but are not limited to: that Tevogen will need to raise additional capital to execute its business plan, which may not be available on acceptable terms or at all; changes in the markets in which Tevogen competes, including with respect to its competitive landscape, technology evolution, or regulatory changes; changes in domestic and global general economic conditions; the risk that Tevogen may not be able to execute its growth strategies or may experience difficulties in managing its growth and expanding operations; the risk that Tevogen may not be able to develop and maintain effective internal controls; the failure to achieve Tevogen’s commercialization and development plans and identify and realize additional opportunities, which may be affected by, among other things, competition, the ability of Tevogen to grow and manage growth economically and hire and retain key employees; the risk that Tevogen may fail to keep pace with rapid technological developments to provide new and innovative products and services or make substantial investments in unsuccessful new products and services; risks related to the ability to develop, license or acquire new therapeutics; the risk of regulatory lawsuits or proceedings relating to Tevogen’s business; uncertainties inherent in the execution, cost, and completion of preclinical studies and clinical trials; risks related to regulatory review, approval and commercial development; risks associated with intellectual property protection; Tevogen’s limited operating history; and those factors discussed or incorporated by reference in Tevogen’s Annual Report on Form 10-K.
You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Tevogen undertakes no obligation to update any forward-looking statements, except as required by applicable law.
Contacts
Tevogen Bio Communications
T: 1 877 TEVOGEN, Ext 701
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/564917d7-587e-4add-85df-d28da482110a









