Tevogen Highlights Potential Role of TVGN 489 in Eliminating Persistent Viral Reservoirs Linked to Long COVID
Rhea-AI Summary
Tevogen Bio (NASDAQ:TVGN) has highlighted the potential of its investigational T cell therapy TVGN 489 in treating Long COVID, which affects an estimated 20 million Americans. Recent peer-reviewed studies have linked Long COVID to persistent viral reservoirs, where SARS-CoV-2 proteins and RNA remain in the body for months to years after infection.
TVGN 489, developed using Tevogen's ExacTcell™ platform, is an off-the-shelf cytotoxic CD8+ T lymphocyte therapy targeting multiple SARS-CoV-2 proteins. In proof-of-concept trials, the therapy demonstrated efficacy in reducing viral load in all patients, with CTLs persisting for at least 6 months without interfering with patients' immune responses.
The company is actively preparing for clinical manufacturing of TVGN 489, which could potentially restore homeostasis in Long COVID patients by eliminating virus-infected cells.
Positive
- Proof-of-concept trial showed TVGN 489 reduced viral load in all patients
- CTLs persisted for 6 months in all tested patients without immune interference
- Therapy targets multiple SARS-CoV-2 proteins, not just Spike protein
- Addresses large market opportunity with 20 million affected Americans
Negative
- Additional research still needed to confirm efficacy
- Clinical manufacturing phase not yet initiated
- Will require additional capital to execute business plan
News Market Reaction
On the day this news was published, TVGNW declined 11.30%, reflecting a significant negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
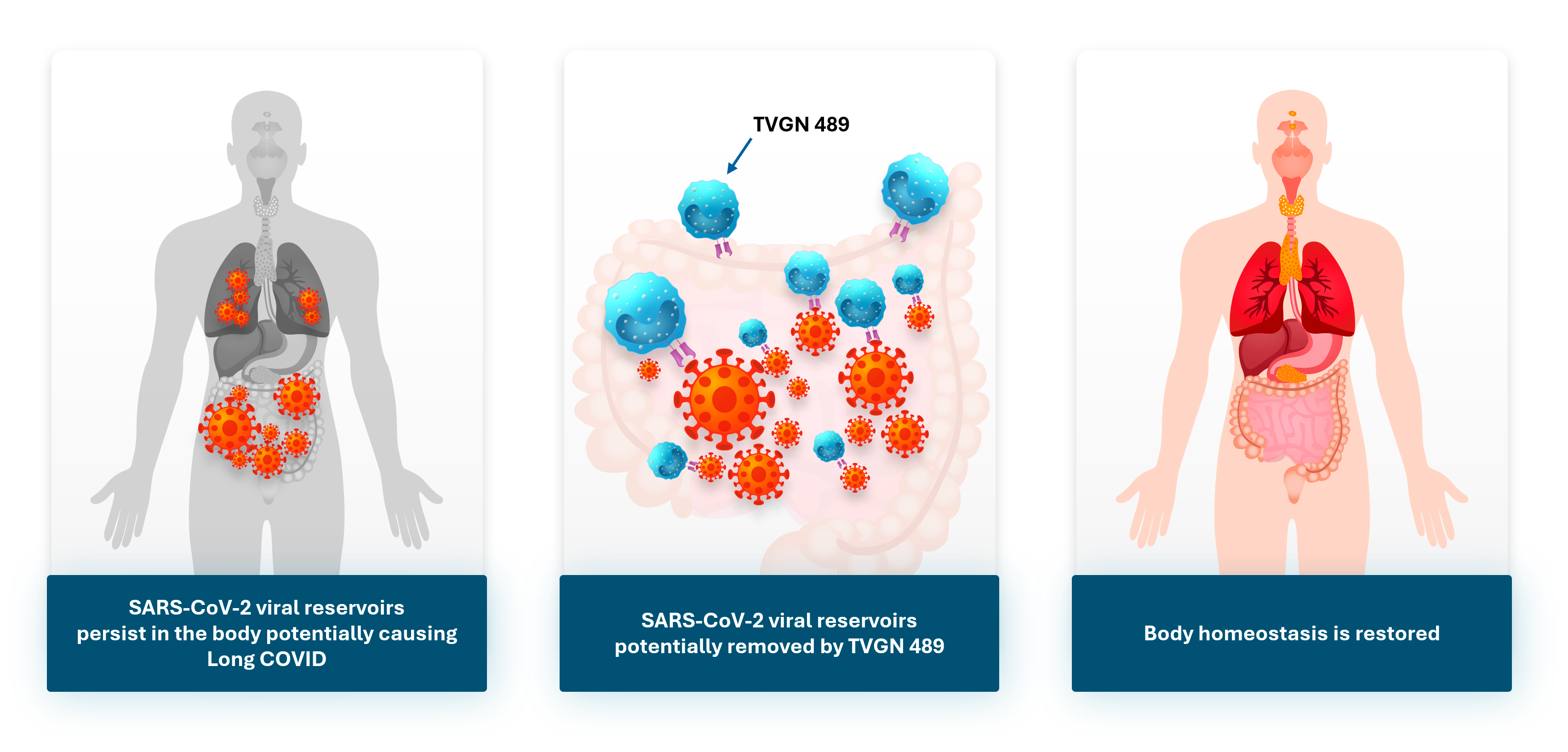
WARREN, N.J., Sept. 23, 2025 (GLOBE NEWSWIRE) -- Tevogen (“Tevogen Bio Holdings Inc.” or “Company”) (Nasdaq: TVGN), today highlights emerging scientific evidence linking persistent viral reservoirs to Long COVID, and emphasizes the potential of its investigational precision T cell therapy, TVGN 489, for the treatment of this debilitating condition which affects an estimated 20 million Americans and represents an area of unmet need.
Peer-reviewed studies such as the ones published in Sci Trans Med, Clin Microbial Infect and The Lancet Infectious Diseases report the detection of residual SARS-CoV-2 proteins and RNA for months to years after acute infection in individuals. This persistence suggests the presence of a viral reservoir which results in chronic immune inflammation and dysfunction underlying Long COVID symptoms. These findings open new avenues for therapeutic approaches aimed at eliminating the residual virus and restoring immune and physiological homeostasis.
Tevogen Bio’s first clinical product, TVGN 489, from its allogeneic precision T cell therapy platform, ExacTcell™, is an off-the-shelf cytotoxic CD8+ T lymphocyte (CTL) therapy designed to target multiple SARS-CoV-2 proteins across the entire viral genome, not just the Spike protein. In Tevogen Bio’s proof-of-concept clinical trial, TVGN 489 showed efficacy in reducing the viral load for all patients and TVGN 489 CTLs persisted for at least 6 months in all patients tested; the CTLs did not interfere with patient’s own immune responses. The Company believes that TVGN 489 may provide broad and durable immune responses against persisting SARS-CoV-2 fragments, including those originating from proteins other than Spike (Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae | Clinical Infectious Diseases | Oxford Academic), potentially representing a strategy for Long COVID treatment.
“These recent scientific reports strengthen the theory that Long COVID is, at least in part, sustained by a persistent viral reservoir,” said Dr. Ryan Saadi, Founder and CEO of Tevogen. “Our investigational therapy, TVGN 489, is uniquely designed to eliminate virus-infected cells. Tevogen is actively preparing for clinical manufacturing of TVGN 489 and while more research is needed, based on positive dose-finding trial data, I am highly optimistic that TVGN 489 has the potential to restore homeostasis in patients suffering from this debilitating condition.”
Forward Looking Statements
This press release contains certain forward-looking statements, including without limitation statements relating to: Tevogen’s plans for its research and manufacturing capabilities; expectations regarding future growth; expectations regarding the healthcare and biopharmaceutical industries; and Tevogen’s development of, the potential benefits of, and patient access to its product candidates for the treatment of infectious diseases and cancer. Forward-looking statements can sometimes be identified by words such as “may,” “could,” “would,” “expect,” “anticipate,” “possible,” “potential,” “goal,” “opportunity,” “project,” “believe,” “future,” and similar words and expressions or their opposites. These statements are based on management’s expectations, assumptions, estimates, projections and beliefs as of the date of this press release and are subject to a number of factors that involve known and unknown risks, delays, uncertainties and other factors not under the company’s control that may cause actual results, performance or achievements of the company to be materially different from the results, performance or other expectations expressed or implied by these forward-looking statements.
Factors that could cause actual results, performance, or achievements to differ from those expressed or implied by forward-looking statements include, but are not limited to: that Tevogen will need to raise additional capital to execute its business plan, which may not be available on acceptable terms or at all; changes in the markets in which Tevogen competes, including with respect to its competitive landscape, technology evolution, or regulatory changes; changes in domestic and global general economic conditions; the risk that Tevogen may not be able to execute its growth strategies or may experience difficulties in managing its growth and expanding operations; the risk that Tevogen may not be able to develop and maintain effective internal controls; the failure to achieve Tevogen’s commercialization and development plans and identify and realize additional opportunities, which may be affected by, among other things, competition, the ability of Tevogen to grow and manage growth economically and hire and retain key employees; the risk that Tevogen may fail to keep pace with rapid technological developments to provide new and innovative products and services or make substantial investments in unsuccessful new products and services; risks related to the ability to develop, license or acquire new therapeutics; the risk of regulatory lawsuits or proceedings relating to Tevogen’s business; uncertainties inherent in the execution, cost, and completion of preclinical studies and clinical trials; risks related to regulatory review, approval and commercial development; risks associated with intellectual property protection; Tevogen’s limited operating history; and those factors discussed or incorporated by reference in Tevogen’s Annual Report on Form 10-K.
You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Tevogen undertakes no obligation to update any forward-looking statements, except as required by applicable law.
Contacts
Tevogen Bio Communications
T: 1 877 TEVOGEN, Ext 701
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/564917d7-587e-4add-85df-d28da482110a








