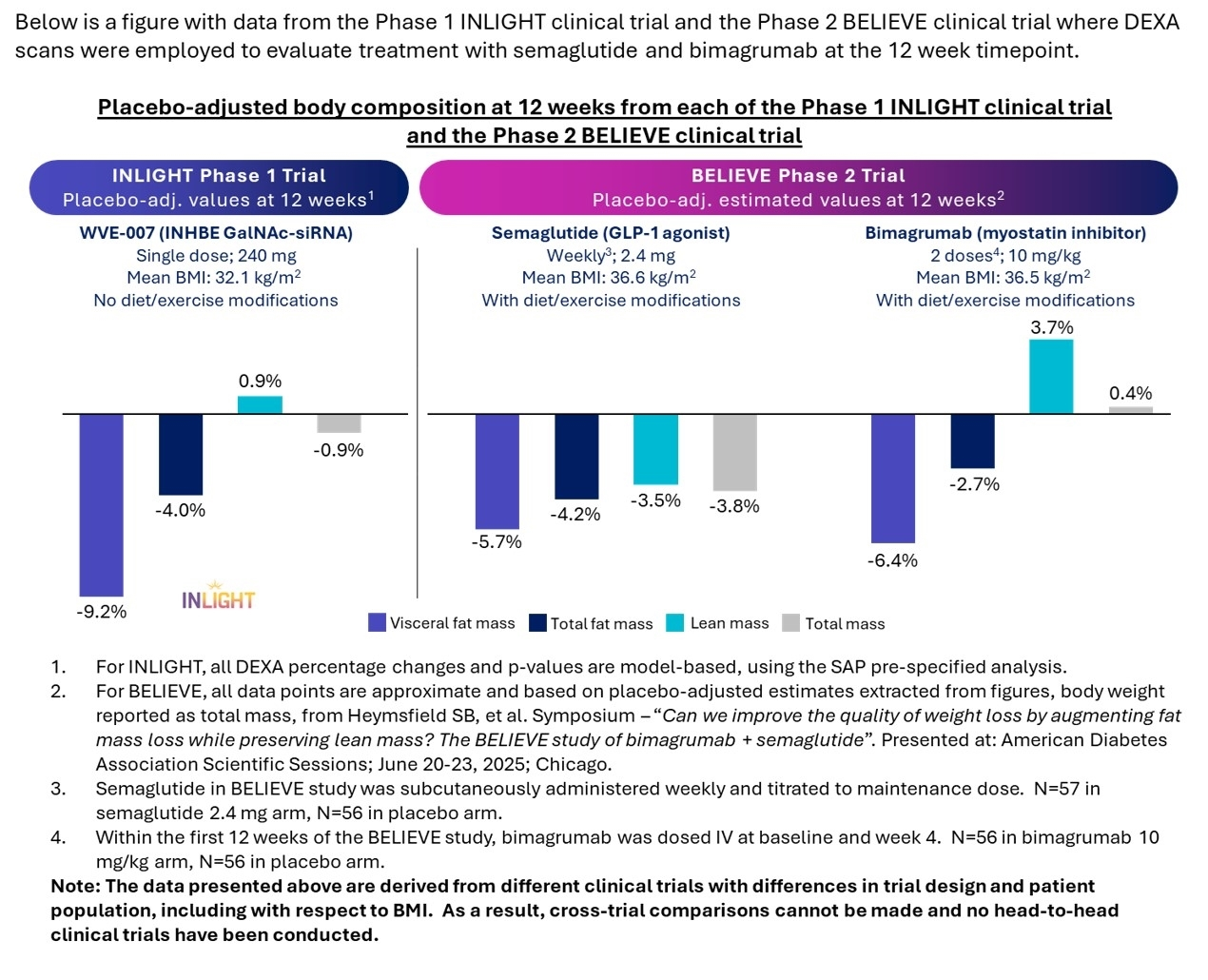
Wave Life Sciences Announces Positive Interim Data from Phase 1 INLIGHT Trial of WVE-007 (INHBE) for Obesity; Single Dose Resulted in Improvement in Body Composition With Fat Loss Similar to GLP-1 at Three Months Without Muscle Loss
Rhea-AI Summary
Wave Life Sciences (Nasdaq: WVE) reported positive interim Phase 1 INLIGHT data for WVE-007 (INHBE) after a single 240 mg subcutaneous dose in individuals with mean baseline BMI ~32 kg/m2.
At Day 85, WVE-007 produced a 9.4% visceral fat reduction (p=0.02), 4.5% total body fat reduction (3.5 lbs; p=0.07), and a 3.2% lean mass increase (4.0 lbs; p=0.01). Mean Activin E fell >75% and maxed at 78% reduction, supporting once- or twice-yearly dosing. Safety was generally favorable up to 600 mg with no serious TEAEs. Phase 2 planning is underway; further 2026 follow-ups expected.
Positive
- Visceral fat reduced by 9.4% at Day 85 (p=0.02)
- Lean mass increased by 3.2% (4.0 lbs) at Day 85 (p=0.01)
- Mean Activin E reductions of >75% sustained to Day 85
- No serious or severe TEAEs; tolerability up to 600 mg
Negative
- Total body fat reduction was 4.5% (3.5 lbs) with p=0.07 (not statistically significant)
- Interim 3-month results; longer-term weight and safety effects unconfirmed beyond Day 85
News Market Reaction
On the day this news was published, WVE gained 147.26%, reflecting a significant positive market reaction. Argus tracked a peak move of +158.3% during that session. Argus tracked a trough of -20.7% from its starting point during tracking. Our momentum scanner triggered 133 alerts that day, indicating very high trading interest and price volatility. This price movement added approximately $2.16B to the company's valuation, bringing the market cap to $3.63B at that time. Trading volume was exceptionally heavy at 56.5x the daily average, suggesting very strong buying interest.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
Peers show mixed, mostly modest moves (e.g., ELVN up 1.77%, PGEN down 5.01%), with no clear, coordinated sector reaction around obesity or RNA therapeutics.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Nov 10 | Earnings and update | Positive | -10.3% | Q3 2025 financials and broad pipeline updates including WVE-007 durability. |
| Nov 07 | Investor conferences | Neutral | -0.3% | Participation in November healthcare investor conferences and obesity panel. |
| Nov 04 | Preclinical obesity data | Positive | +0.4% | Preclinical WVE-007 obesity data and early INLIGHT clinical biomarker results. |
| Nov 04 | Earnings scheduling | Neutral | -3.6% | Announcement of timing for the Q3 2025 earnings call and webcast. |
| Oct 29 | INLIGHT target data | Positive | +8.8% | Positive INLIGHT target engagement and Activin E reduction data for WVE-007. |
Positive clinical data for WVE, including prior WVE-007 updates, has often coincided with meaningful upside moves, while broader business or earnings updates have sometimes seen negative reactions.
Over recent months, Wave highlighted multiple milestones across its RNA pipeline. Clinical updates, including INLIGHT WVE-007 obesity data on Oct 29, 2025 and other trials like WVE-N531 in DMD, were followed by share gains of ~6–9%. In contrast, the Q3 2025 earnings release on Nov 10, 2025 coincided with a -10.31% move. Today’s Phase 1 INLIGHT body-composition data extends the earlier Activin E and target-engagement story for WVE-007.
Market Pulse Summary
The stock surged +147.3% in the session following this news. A strong positive reaction aligns with prior clinical-trial news for Wave, where WVE-007 and other programs have driven moves of around 6–9%. The body-composition data extend earlier Activin E reductions with a 9.4% visceral fat drop and lean-mass gain. Investors would still need to weigh execution through upcoming Phase 2 plans and the risk that enthusiasm exceeds what early Phase 1 data justify.
Key Terms
galnac-sirna medical
subcutaneous medical
dexA medical
hba1c medical
bmi medical
teaes medical
AI-generated analysis. Not financial advice.
Following a single subcutaneous 240 mg dose, WVE-007 (INHBE GalNAc-siRNA) improved body composition at three months compared to baseline, with a
Sustained and robust suppression of serum Activin E supports expectations for continued improvements in body composition, further fat loss, and preserved muscle, with once or twice-yearly dosing
Generally safe and well tolerated with only mild treatment related adverse events and no clinically meaningful changes in clinical laboratory measurements, including lipid profiles or liver function tests
Planning underway for Phase 2 trials evaluating WVE-007 both as a monotherapy and an add-on therapy to incretins in populations with higher BMI and related co-morbidities, and as maintenance post-incretin treatment
Further clinical data updates expected in 1Q 2026, including six-month follow-up from the 240 mg single-dose cohort and three-month follow-up from 400 mg single-dose cohort
Investor conference call and webcast at 8:30 a.m. ET today
CAMBRIDGE, Mass., Dec. 08, 2025 (GLOBE NEWSWIRE) -- Wave Life Sciences Ltd. (Nasdaq: WVE), a clinical-stage biotechnology company focused on unlocking the broad potential of RNA medicines to transform human health, today announced positive interim data from the lowest therapeutic cohort of the ongoing first-in-human INLIGHT trial evaluating WVE-007, an investigational INHBE GalNAc-siRNA using Wave’s proprietary SpiNA design, for the treatment of obesity. In this interim assessment, a single 240 mg dose of WVE-007 led to an improvement in body composition characterized by reductions in total and visceral fat mass at three months and an increase in lean mass. There was also a favorable safety profile as well as durable reductions in serum Activin E that support potential once or twice-yearly dosing.
These results add to a growing body of data supporting the silencing of INHBE and its downstream protein product (Activin E) as a therapeutic approach to obesity with strong evidence from human genetics. Individuals who have a protective loss-of-function variant in one copy of the INHBE gene have a healthier body composition and cardiometabolic profile, including less visceral fat and lower risk of developing type 2 diabetes and cardiovascular disease. Importantly, visceral fat on its own is closely associated with many diseases, including cardiometabolic disorders.
“Today’s update demonstrates the tremendous potential of WVE-007 to transform the obesity treatment paradigm, addressing the biggest disadvantages of GLP-1s: fat loss at the expense of muscle, poor tolerability, including GI and other side effects, along with frequent dosing. After only three months at our lowest therapeutic single dose of WVE-007, we are observing fat loss that exceeded our expectations and is on par with GLP-1s without their associated impact on muscle loss, and a compelling safety and tolerability profile. Our data also continue to support the potential for once or twice a year dosing. We have multiple opportunities in the near-term to assess the impact of longer exposure and higher doses on fat loss, lean mass preservation, and body weight change,” said Paul Bolno, MD, MBA, President and Chief Executive Officer at Wave Life Sciences. “These data affirm WVE-007’s significant potential to provide a meaningful treatment for the over one billion people living with obesity, as a once to twice-yearly monotherapy, add-on therapy to incretins, or maintenance therapy post incretin treatment, and planning for Phase 2 trials in these settings is underway.”
The INLIGHT clinical trial is a randomized, placebo-controlled (3:1) Phase 1 study of otherwise healthy individuals living with overweight or obesity, BMI between 28 and 35 kg/m2, and HbA1c of less than 5.9. The study does not include any diet or exercise modifications. As of the data cut-off date, INLIGHT has enrolled over 100 individuals. Today’s update includes the three-month follow-up from the single subcutaneous 240 mg dose cohort in 32 individuals with an approximate mean baseline BMI of 32 kg/m2. Results reported are from the prespecified statistical analysis plan.
From baseline, a single dose of WVE-007 improved body composition and led to a
Consistent and durable serum Activin E reductions continue to be observed across participants and support WVE-007’s potential for once or twice-yearly dosing. Maximum reductions in serum Activin E of
WVE-007 continues to be generally safe and well tolerated to date up to 600 mg. There were no discontinuations, and no severe or serious treatment emergent adverse events (TEAEs). All TEAEs were mild or moderate, and all treatment-related adverse events were mild. There were no clinically meaningful changes in clinical laboratory measurements, including lipid profiles or liver function tests.
“I am encouraged by Wave’s early clinical results, which suggest a promising new approach to obesity and metabolic health treatment. The data indicate meaningful reductions in body fat, particularly targeting harmful visceral fat, while preserving lean mass,” said Angela Fitch, MD, FACP, co-founder and Chief Medical Officer at knownwell, former co-director of the Massachusetts General Hospital Weight Center, faculty at Harvard Medical School, and former president of the Obesity Medicine Association. “A therapy delivered once or twice a year has the potential to dramatically improve access, adherence, and long-term outcomes. In clinical practice, we emphasize that the goal is to become leaner, not simply lighter. Obesity treatment is about health gains, not weight loss, and Wave’s emerging therapy has the potential to make these goals a reality in a way that could fundamentally reshape longitudinal obesity and cardiometabolic disease care.”
Anticipated upcoming clinical updates
The INLIGHT clinical trial is ongoing with the 240 mg (n=32), 400 mg (n=32), and 600 mg (n=32) cohorts fully dosed. In the first quarter of 2026, Wave expects to deliver further follow-up (six-month) from the 240 mg single-dose cohort, as well as three-month follow-up data from the 400 mg single-dose cohort. In the second quarter of 2026, Wave expects to deliver six-month follow-up data from the 400 mg single-dose cohort and three-month follow-up data from the 600 mg single-dose cohort.
Wave is actively planning for Phase 2 trials evaluating WVE-007 as both a monotherapy and an add-on therapy to incretins in populations with higher BMI and related co-morbidities, and as a maintenance therapy post-incretin treatment.
Investor Conference Call and Webcast
Wave will host an investor conference call today at 8:30 a.m. ET to review the INLIGHT Phase 1 interim clinical data. A webcast of the conference call can be accessed by visiting “Investor Events” on the investor relations section of the Wave Life Sciences website: https://ir.wavelifesciences.com/events-publications/events. Analysts planning to participate during the Q&A portion of the live call can join the conference call at the audio-conferencing link here. Once registered, participants will receive the dial-in information. Following the live event, an archived version of the webcast will be available on the Wave Life Sciences website.
About WVE-007
WVE-007 is an investigational GalNAc-siRNA that utilizes Wave’s best-in-class proprietary oligonucleotide chemistry and the company’s Stereopure interfering Nucleic Acid (SpiNA) next generation siRNA design. WVE-007 is designed to silence INHBE mRNA, an obesity target with strong evidence from human genetics. Individuals who have a protective loss-of-function variant in one copy of the INHBE gene have a healthier body composition and cardiometabolic profile, including less visceral fat and lower risk of type 2 diabetes or cardiovascular disease. In preclinical models, INHBE GalNAc-siRNA led to adipocyte shrinkage, fewer pro-inflammatory macrophages, less fibrosis, and improved insulin sensitivity in visceral adipose tissue, supporting potential for metabolic improvement. As an add-on to semaglutide, Wave’s GalNAc-siRNA doubled weight loss in mice and prevented weight regain upon cessation of semaglutide.
About the INLIGHT Clinical Trial
INLIGHT is an ongoing, first-in-human clinical trial (3:1 active: placebo) evaluating WVE-007 in adults living with overweight or obesity and assesses safety, tolerability, pharmacokinetics, Activin E, body weight and composition, and biomarkers of metabolic health. INLIGHT is currently ongoing at multiple trial sites, including in the US.
About Wave Life Sciences
Wave Life Sciences (Nasdaq: WVE) is a biotechnology company focused on unlocking the broad potential of RNA medicines to transform human health. Wave’s RNA medicines platform, PRISM®, combines multiple modalities, chemistry innovation and deep insights in human genetics to deliver scientific breakthroughs that treat both rare and common disorders. Its toolkit of RNA-targeting modalities includes editing, splicing, RNA interference, and antisense silencing, providing Wave with unmatched capabilities for designing and sustainably delivering candidates that optimally address disease biology. Wave’s diversified pipeline includes clinical programs in obesity, alpha-1 antitrypsin deficiency, Duchenne muscular dystrophy, and Huntington’s disease, as well as several preclinical programs utilizing the company’s broad RNA therapeutics toolkit. Driven by the calling to “Reimagine Possible,” Wave is leading the charge toward a world in which human potential is no longer hindered by the burden of disease. Wave is headquartered in Cambridge, MA. For more information on Wave’s science, pipeline and people, please visit www.wavelifesciences.com and follow Wave on X and LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements concerning our goals, beliefs, expectations, strategies, objectives and plans, and other statements that are not necessarily based on historical facts, including statements regarding the following, among others: the anticipated initiation, site activation, patient recruitment, patient enrollment, dosing, generation and reporting of data and completion of our clinical trials, including interactions with regulators and any potential registration based on these data, and the timing and announcement of such events; our understanding of the dose levels and dosing frequency of our therapeutic candidates; our understanding of the safety profile of our therapeutic candidates; our expectations for our clinical candidates and the anticipated therapeutic benefits thereof; the potential of WVE-007’s mechanism (INHBE GalNAc-siRNA) as a novel and unique obesity treatment to improve body composition, induce fat loss, preserve muscle, and drive weight loss; the potential for WVE-007 as both a monotherapy and an add-on therapy to incretins in populations with higher BMI and related co-morbidities, and as maintenance post-incretin treatment; the anticipated benefits of our therapeutic candidates and pipeline, as well as status and progress of our programs relative to potential competitors, including WVE-007 and its potential to improve metabolic health and transform the obesity treatment paradigm; the protocol, design and endpoints of our clinical trials; the future performance and results of our programs in clinical trials; our expectations with respect to how our preclinical and clinical data successes to date may predict the behavior of our compounds in humans, predict success for our future therapeutic candidates, future clinical data readouts and further validate of our platform; patient population estimates related to our therapeutic candidates and the potential addressable market that our therapeutics may address; our ability to design compounds using various modalities and the anticipated benefits of that approach; the breadth and versatility of our PRISM drug discovery and development platform; the expected benefits of our stereopure oligonucleotides compared with stereorandom oligonucleotides; the potential benefits of our RNA editing capability, including our AIMers, compared to others; the potential benefits of our emerging pipeline of siRNAs (SpiNA) compared to others; the benefits of RNA medicines generally; the potential for certain of our programs to be best-in-class or first-in-class; our ability to translate genetic insights into high impact medicines; our expectations on the company’s future growth. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this press release are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual results to differ materially from those indicated by these forward-looking statements as a result of these risks, uncertainties and important factors, including, without limitation, the risks and uncertainties described in the section entitled “Risk Factors” in Wave’s most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission (SEC), as amended, and in other filings Wave makes with the SEC from time to time. Wave undertakes no obligation to update the information contained in this press release to reflect subsequently occurring events or circumstances.
Contact:
Kate Rausch
VP, Corporate Affairs and Investor Relations
+1 617-949-4827
Investors:
James Salierno
Director, Investor Relations
+1 617-949-4043
InvestorRelations@wavelifesci.com
Media:
Katie Sullivan
Senior Director, Corporate Communications
+1 617-949-2936
MediaRelations@wavelifesci.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/69d657dd-9345-4780-a041-7271c9a59ff6








