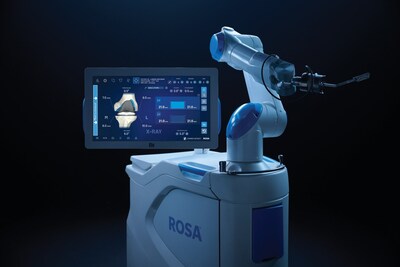
Zimmer Biomet Receives U.S. FDA Clearance for Enhanced Version of ROSA® Knee Robotic Technology
Rhea-AI Summary
Zimmer Biomet (NYSE: ZBH) announced U.S. FDA 510(k) clearance of ROSA® Knee with OptimiZe™, an enhanced version of its ROSA Knee robotic system for total knee replacement surgery.
The update adds five OptimiZe features—Planning, Landmarking, Tracking, Kinematic Alignment and Experience—plus integration with ZBEdge® Analytics. The company said OptimiZe Planning™ can reduce planning time by an average of 46%. Zimmer Biomet plans a targeted release later in 2025 and expects U.S. commercial availability in Q1 2026.
Positive
- FDA 510(k) clearance for ROSA Knee with OptimiZe
- 46% average reduction in planning time (OptimiZe Planning™)
- Five new OptimiZe features enhancing planning, tracking, alignment
- Integration with ZBEdge Analytics for intra-operative data-driven decisions
- U.S. commercial rollout expected in Q1 2026
Negative
- Initial distribution is a targeted release, limiting near-term scale
- Clearance is 510(k) rather than a full premarket approval pathway
News Market Reaction
On the day this news was published, ZBH declined 0.73%, reflecting a mild negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
ROSA ® Knee with OptimiZe ™ Offers Personalized Surgical Planning and Drives Confidence in Delivering Accurate and Reproducible Outcomes1
WARSAW, Ind., Nov. 14, 2025 /PRNewswire/ -- Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global medical technology leader, today announced
ROSA Knee with OptimiZe personalizes the surgeon's experience with customized intelligent surgical planning and new positioning, tracking and alignment features to help ensure accuracy1 and reduce user variability.2 The technology provides a simplified user interface that allows surgeons to choose the information they want to see, when they want to see it.
"More and more surgeons are integrating robotic technologies, because they empower surgeons to achieve better outcomes for patients3," said Dr. Peter Sculco, hip and knee replacement surgeon at Hospital for Special Surgery in
Designed for use with the industry leading Persona® Knee System4, ROSA Knee with OptimiZe enables surgeons who use functional alignment to use customizable surgeon profiles that systematically provide surgical plans to automatically position the implant and balance the knee based on the patient's own anatomy and the surgeon's preferences. For surgeons who prefer kinematic alignment, the system offers the industry's only automated kinematic alignment feature to resurface the knee with the goal of restoring its pre-arthritic position and native joint lines.
"As a leader in advancing innovation in orthopedic robotics, we are committed to improving and enhancing our robotic technologies to better meet the needs of surgeons and improve efficiency in the OR, with the ultimate goal of delivering better outcomes for patients," said Shaun Braun, senior vice president and chief information and technology officer at Zimmer Biomet. "ROSA Knee with OptimiZe was designed in partnership with our seasoned team of ROSA surgeons to make robotic-assisted knee replacement surgery more personalized, accurate and efficient for surgeons. With our proprietary algorithm, OptimiZe Planning™, surgeons can create customized profiles that generate personalized surgical plans, which can reduce planning time by an average of
ROSA Knee with OptimiZe features five key enhancements to the ROSA Knee System:
- OptimiZe Planning™: Creates a customized surgical plan to guide implant positioning based on surgeon individual knee balancing preferences.
- OptimiZe Landmarking™: Easy-to-use painting feature reduces user landmarking variability.
- OptimiZe Tracking™: Collaborative resections with motion-sensitive Active Track™ eliminate the need for pinning the Cut Guide to the bone and allows bony resections to remain on plane even with leg movement.
- OptimiZe Kinematic Alignment™: Automated kinematic alignment plan based on bony landmarks, to resurface the knee to its pre-arthritic position.
- OptimiZe Experience™: Simplified user interface allows the surgeon to choose specific workflow and display options to tailor user experience for every case.
ROSA Knee with OptimiZe integrates with ZBEdge® Analytics to enable surgeons to make data-driven intra-operative decisions, objectively assess their performance and understand the potential impact of clinical decisions on patient recovery.
Zimmer Biomet will conduct a targeted release of ROSA Knee with OptimiZe later this year with commercial availability in the
About Zimmer Biomet
Zimmer Biomet is a global medical technology leader with a comprehensive portfolio designed to maximize mobility and improve health. We seamlessly transform the patient experience through our innovative products and suite of integrated digital and robotic technologies that leverage data, data analytics and artificial intelligence.
With 90+ years of trusted leadership and proven expertise, Zimmer Biomet is positioned to deliver the highest quality solutions to patients and providers. Our legacy continues to come to life today through our progressive culture of evolution and innovation.
For more information about our product portfolio, our operations in 25+ countries and sales in 100+ countries or about joining our team, visit www.zimmerbiomet.com or follow on LinkedIn at www.linkedin.com/company/zimmerbiomet or X at www.x.com/zimmerbiomet.
Cautionary Statement Regarding Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, but are not limited to, statements concerning Zimmer Biomet's expectations, plans, prospects, and product and service offerings, including new product launches and potential clinical successes. Such statements are based upon the current beliefs and expectations of management and are subject to significant risks, uncertainties and changes in circumstances that could cause actual outcomes and results to differ materially. For a list and description of some of such risks and uncertainties, see Zimmer Biomet's periodic reports filed with the
|
Contacts: Media Troy Kirkpatrick 614-284-1926 troy.kirkpatrick@zimmerbiomet.com
Kirsten Fallon 781-779-5561 |
Investors David DeMartino 646-531-6115 david.demartino@zimmerbiomet.com
Zach Weiner 908-591-6955 |
1 Data on File. DVaR-DS250106-01 ROSA Knee System v1.5 Validation Report.
2 Data on file. FER-EMS230714-01 Formative Evaluation Report – July Lab 2023.
3 Ponna AK, Giakas AM, Khoudary AA, Siddiqi A. Advancements in Robotic Orthopaedic Surgery: A Current Concept. SurgiColl. 2025;3(1). doi:10.58616/001c.132487.
4 IQVIA Sales Data.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/zimmer-biomet-receives-us-fda-clearance-for-enhanced-version-of-rosa-knee-robotic-technology-302615106.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/zimmer-biomet-receives-us-fda-clearance-for-enhanced-version-of-rosa-knee-robotic-technology-302615106.html
SOURCE Zimmer Biomet Holdings, Inc.