Zomedica Launches Enhanced Equine Insulin Assay for the TRUFORMA(R) Diagnostic Platform Featuring an Extended Range and Automatic Sample Dilution
Rhea-AI Summary
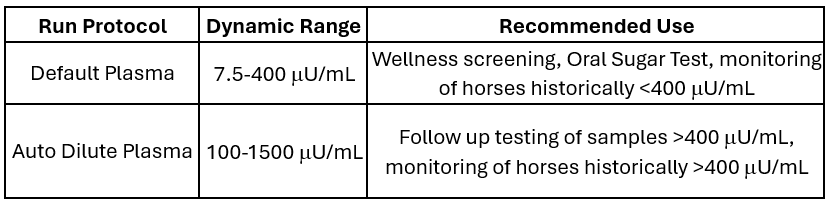
Zomedica (OTCQB:ZOMDF) has announced significant enhancements to its equine insulin assay for the TRUFORMA Diagnostic Platform. The update features an extended dynamic range, increased by 60% from its previous version, and introduces an innovative automatic sample dilution function. This advancement allows veterinarians to measure insulin levels at the highest ranges without additional steps or increased time-to-result.
The new automatic dilution feature eliminates the need for manual dilution protocols for samples exceeding 250 µU/mL, streamlining the testing process. The enhancement maintains test consistency while freeing staff from additional tasks. This development positions TRUFORMA as having the widest dynamic range available both at Point of Care and compared to Reference Labs.
Positive
- Introduction of automatic sample dilution feature eliminates manual steps and saves time
- 60% increase in the dynamic range of the standard insulin test
- Maintains industry-leading position with widest dynamic range in both Point of Care and Reference Lab settings
- Growing market presence indicated by being one of their fastest-growing assays
Negative
- None.
Insights
Zomedica's enhanced equine insulin assay offers significant competitive advantages through automatic dilution and expanded testing range.
Zomedica's update to their equine insulin assay represents a significant technological advancement in point-of-care veterinary diagnostics. The enhancement addresses a critical clinical need in equine medicine, as insulin dysregulation is a primary risk factor for laminitis, a serious condition in horses.
The two key improvements deliver substantial value:
- A 60% increase in the default dynamic range of the test
- Automatic sample dilution capability within the existing cartridge system
The automatic dilution feature is particularly noteworthy because it eliminates workflow inefficiencies while ensuring result consistency. Traditional methods require veterinarians to manually dilute samples and rerun tests when insulin levels exceed upper detection limits, which introduces variability and extends time-to-result.
This platform enhancement demonstrates Zomedica's responsive product development approach, as it was implemented following practitioner feedback from the original October 2024 release. The modification capitalizes on the unique single-use cartridge design of the TRUFORMA platform, creating a competitive advantage that, according to the company, other systems cannot match.
From a market perspective, this improvement targets a growing clinical concern, as insulin dysregulation testing in equines has gained importance in preventing potentially catastrophic laminitis cases. By offering immediate, expanded-range results at the point of care, Zomedica has positioned its technology to potentially capture market share from reference laboratories that previously handled high-concentration samples.
The TRUFORMA Platform now has the widest dynamic range (without manual dilution) available either at the Point of Care or from a Reference Lab
ANN ARBOR, MI / ACCESS Newswire / May 21, 2025 / Zomedica Corp. (OTCQB:ZOMDF) ("Zomedica" or the "Company"), a veterinary health company offering point-of-care diagnostics and therapeutic products for equine and companion animals, today announced an update to one of their fastest growing assays, insulin for equine plasma. This update increases the test's already industry-leading point of care dynamic range and adds a new function, automatic sample dilution, that allows a veterinarian to measure insulin at the highest levels with no additional steps or increase in time-to-result on the TRUFORMA In-Clinic Biosensor Testing Platform.
"When we introduced our equine insulin assay in October of 2024, one of the most frequent requests we received was for a dilution protocol to measure insulin level in horses suspected to be greater than 250 µU/mL," said Ian Harmon, Senior Director, R&D. Ian continues, "In response, rather than validating an external dilution protocol that would involve additional steps for the veterinarian and staff, our engineers designed a way to have the TRUFORMA device automatically dilute the sample for them and run the test with no additional time or steps. The user simply selects the 'Auto Dilute' option from the test menu screen, and the TRUFORMA device takes it from there. The uniqueness of our single-use test cartridges allows us this flexibility of design and is something that no other platform can do."
By having the TRUFORMA device perform the dilution automatically on the existing test cartridge, consistency from test-to-test is assured and the staff is freed from having to perform a tedious additional task.
The Zomedica R&D team was also able to increase the already industry-leading dynamic range of the standard (or default, undiluted) in-clinic insulin test by
T.J. Barclay, DVM, Senior Professional Services Veterinarian for Zomedica, commented, "In recent years, insulin dysregulation in horses and ponies has been increasingly recognized as the most significant risk factor for developing laminitis. In the more severe cases, insulin levels may be above a test's upper limit of detection, rendering us unable to determine by serial monitoring if our treatment is effective. In those cases, quantifying extremely high insulin levels has required sending samples to a reference lab where they can perform sample dilutions. Having a device that gives us results in minutes at the point of care that has a both a high dynamic range by default and a process that dilutes the sample on demand covers all patient types we may encounter, helping us make quicker decisions during the treatment and monitoring process."
With the increased dynamic range and new automatic dilution function, along with the consistency, accuracy, and convenience of the original assay, Zomedica has set a new standard for point of care equine insulin testing.
The TRUFORMA Insulin assay for equine plasma with automatic sample dilution may be ordered now from Zomedica. For more information, visit www.zomedica.com.
About Zomedica
Zomedica is a leading equine and companion animal healthcare company dedicated to improving animal health by providing veterinarians with innovative therapeutic and diagnostic solutions. Our gold standard PulseVet® shock wave system, which accelerates healing in musculoskeletal conditions, has transformed veterinary therapeutics. Our suite of products also includes the Assisi® Loop line of therapeutic devices and the TRUFORMA® diagnostic platform, the TRUVIEW® digital cytology system, and the VetGuardian® no-touch monitoring system, all designed to empower veterinarians to provide top-tier care. In the aggregate, their total addressable market in the U.S. exceeds
Follow Zomedica
Email Alerts: http://investors.zomedica.com
Facebook: https://m.facebook.com/zomedica
X (formerly Twitter): https://twitter.com/zomedica
Instagram: https://www.instagram.com/zomedica_inc
Cautionary Note Regarding Forward-Looking Statements
Except for statements of historical fact, this news release contains certain "forward-looking information" or "forward-looking statements" (collectively, "forward-looking information") within the meaning of applicable securities law. Forward-looking information is frequently characterized by words such as "plan", "expect", "project", "intend", "believe", "anticipate", "estimate" and other similar words, or statements that certain events or conditions "may" or "will" occur and include statements relating to our expectations regarding future results. Although we believe that the expectations reflected in the forward-looking information are reasonable, there can be no assurance that such expectations will prove to be correct. We cannot guarantee future results, performance, or achievements. Consequently, there is no representation that the actual results achieved will be the same, in whole or in part, as those set out in the forward-looking information.
Forward-looking information is based on the opinions and estimates of management at the date the statements are made, including assumptions with respect to economic growth, demand for the Company's products, the Company's ability to produce and sell its products, sufficiency of our budgeted capital and operating expenditures, the satisfaction by our strategic partners of their obligations under our commercial agreements and our ability to realize upon our business plans and cost control efforts.
Our forward-looking information is subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those anticipated in the forward-looking information. Some of the risks and other factors that could cause the results to differ materially from those expressed in the forward-looking information include, but are not limited to: the outcome of clinical studies, the application of generally accepted accounting principles, which are highly complex and involve many subjective assumptions, estimates, and judgments, uncertainty as to whether our strategies and business plans will yield the expected benefits; uncertainty as to the timing and results of development work and verification and validation studies; uncertainty as to the timing and results of commercialization efforts, including international efforts, as well as the cost of commercialization efforts, including the cost to develop an internal sales force and manage our growth; uncertainty as to our ability to realize the anticipated growth opportunities from our acquisitions; uncertainty as to our ability to supply products in response to customer demand; supply chain risks associated with tariff changes;; uncertainty as to the likelihood and timing of any required regulatory approvals, and the availability and cost of capital; the ability to identify and develop and achieve commercial success for new products and technologies; veterinary acceptance of our products and purchase of consumables following adoption of our capital equipment; competition from related products; the level of expenditures necessary to maintain and improve the quality of products and services; changes in technology and changes in laws and regulations; our ability to secure and maintain strategic relationships; performance by our strategic partners of their obligations under our commercial agreements, including product manufacturing obligations; risks pertaining to permits and licensing, intellectual property infringement risks, risks relating to any required clinical trials and regulatory approvals, risks relating to the safety and efficacy of our products, the use of our products, intellectual property protection, and the other risk factors disclosed in our filings with the SEC and under our profile on SEDAR+ at www.sedarplus.com. Readers are cautioned that this list of risk factors should not be construed as exhaustive.
The forward-looking information contained in this news release is expressly qualified by this cautionary statement. We undertake no duty to update any of the forward-looking information to conform such information to actual results or to changes in our expectations except as otherwise required by applicable securities legislation. Readers are cautioned not to place undue reliance on forward-looking information.
Investor Relations Contact:
Zomedica Investor Relations
investors@zomedica.com
1-734-369-2555
SOURCE: Zomedica Corp.
View the original press release on ACCESS Newswire







