Apellis Announces 5-Year GALE Data Showing SYFOVRE® (pegcetacoplan injection) Delayed Progression of Geographic Atrophy by ~1.5 Years
Rhea-AI Summary
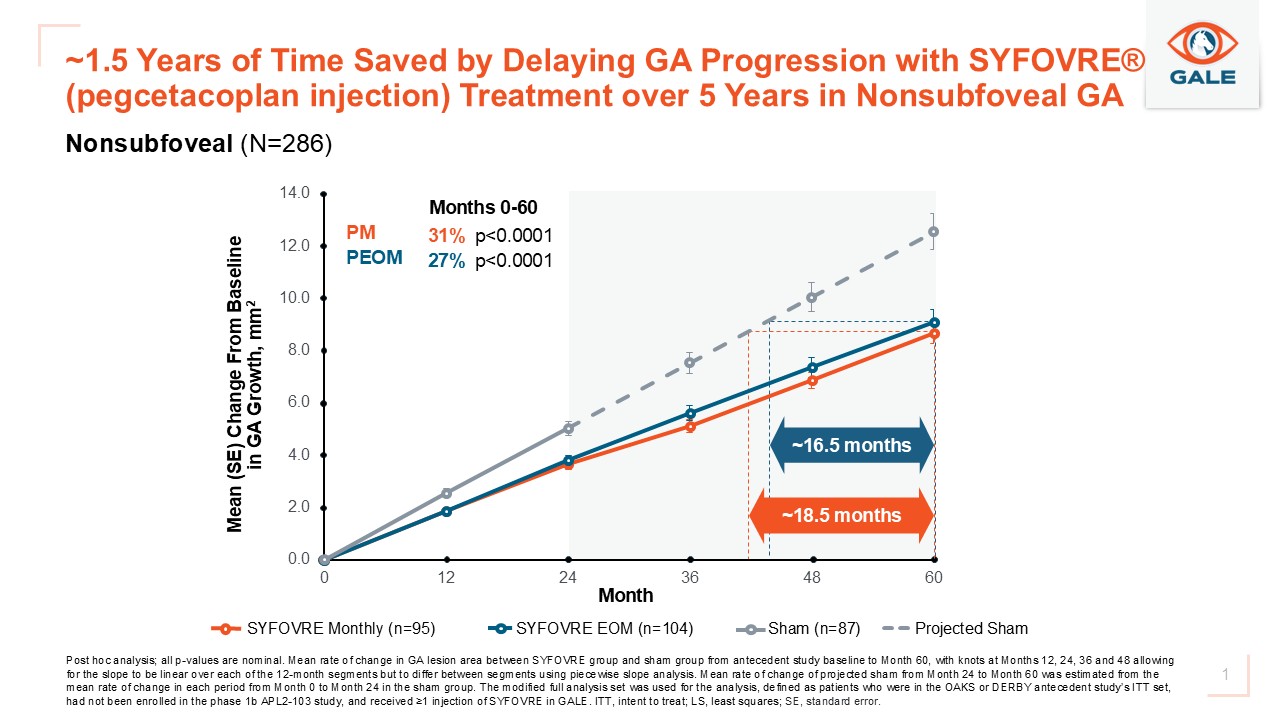
Apellis (Nasdaq: APLS) reported five-year post hoc GALE data showing continuous treatment with SYFOVRE (pegcetacoplan) delayed geographic atrophy (GA) lesion growth by approximately 1.5 years versus sham/projected sham in patients with nonsubfoveal GA. Both monthly and every-other-month dosing regimens showed similar delay. The safety profile through five years remained consistent with prior reports. Detailed results will be presented at a future medical meeting.
Positive
- GA progression delayed ~1.5 years (5-year follow-up)
- Both monthly and EOM dosing showed similar effect
- Five-year continuous treatment dataset available
- Safety profile remained consistent through five years
Negative
- Result type was a post hoc analysis, not a primary endpoint
- Effect reported only in nonsubfoveal GA subgroup
- Detailed readout pending presentation; full data not yet public
News Market Reaction – APLS
On the day this news was published, APLS gained 2.61%, reflecting a moderate positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
WALTHAM, Mass., Nov. 12, 2025 (GLOBE NEWSWIRE) -- Apellis Pharmaceuticals, Inc. (Nasdaq: APLS) today announced data from a post hoc analysis of the GALE extension study following five years of continuous treatment with SYFOVRE® (pegcetacoplan injection), the leading treatment for geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
The results demonstrate that both every-other-month and monthly SYFOVRE delayed GA lesion growth by approximately 1.5 years in patients with nonsubfoveal GA when compared to sham/projected sham.
“I’m very encouraged by these long-term results, which show that early and continuous treatment with SYFOVRE can meaningfully delay the progression of GA,” said Dilsher Dhoot, M.D., California Retina Consultants. “Importantly, these data indicate that SYFOVRE alters the natural course of this disease, which causes irreversible vision loss and profoundly impacts patients’ daily lives.”
“These five-year results underscore the transformative and durable impact of targeting C3 with SYFOVRE to delay the progression of GA,” said Caroline Baumal, M.D., chief medical officer, Apellis. “With the most extensive data set in GA, our broad clinical and real-world experience has greatly advanced the retina community’s understanding of this devastating disease and reinforced Apellis’ leadership.”
The safety profile of SYFOVRE through five years remained consistent with previously reported data. Detailed results will be presented at a future medical meeting.
About the GALE Long-Term Extension Study
GALE (n=792) is a Phase 3, multicenter, open-label, extension study to evaluate the long-term efficacy and safety of SYFOVRE® (pegcetacoplan injection) in patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD). The objectives of the study are to evaluate the long-term incidence and severity of ocular and systemic treatment emergent adverse events as well as change in the total area of GA lesions as measured by fundus autofluorescence. More than 80 percent of participants who completed the OAKS and DERBY studies entered the GALE study.
Patients included in the 5-year GALE lesion growth reduction analyses were in the SYFOVRE treatment arms through Month 24 in the OAKS and DERBY studies and remained on the same regimen in GALE. Sham-treated patients in the Phase 3 OAKS and DERBY studies were eligible to transition to SYFOVRE treatment in GALE after Month 24, so a projected sham arm was used to estimate the growth of GA lesions without treatment between Months 24 and 60. The projected sham arm is based on the observed linear growth of GA lesions over two years and was validated by a fellow eye analysis. It was estimated as the average 12-month mean rate of change in the OAKS and DERBY sham arms through Month 24.
About the Phase 3 OAKS and DERBY Studies
OAKS (n=637) and DERBY (n=621) were Phase 3, multicenter, randomized, double-masked, sham-controlled studies that compared the efficacy and safety of SYFOVRE® (pegcetacoplan injection) with sham injections across a broad and heterogenous population of patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD). The studies evaluated the efficacy of monthly and every-other-month SYFOVRE in patients with GA assessed by change in the total area of GA lesions from baseline as measured by fundus autofluorescence.
In these studies at 24 months, both every-other-month and monthly SYFOVRE reduced GA lesion growth with increasing effects over time and a well-demonstrated safety profile.
About SYFOVRE® (pegcetacoplan injection)
SYFOVRE® (pegcetacoplan injection) is the first-ever approved therapy for geographic atrophy (GA). By targeting C3, SYFOVRE is designed to provide comprehensive control of the complement cascade, part of the body’s immune system. SYFOVRE is approved in the United States for the treatment of GA secondary to age-related macular degeneration.
About Geographic Atrophy (GA)
Geographic atrophy (GA) is an advanced form of age-related macular degeneration and a leading cause of blindness worldwide, impacting more than one million Americans and five million people worldwide.1,2 It is a progressive and irreversible disease caused by the growth of lesions, which destroy the retinal cells responsible for vision. The vision loss caused by GA severely impairs independence and quality of life by making it difficult to participate in daily activities. On average, it takes only 2.5 years for GA lesions to start impacting the fovea, which is responsible for central vision.3
U.S. Important Safety Information for SYFOVRE® (pegcetacoplan injection)
CONTRAINDICATIONS
- SYFOVRE is contraindicated in patients with ocular or periocular infections, in patients with active intraocular inflammation, and in patients with hypersensitivity to pegcetacoplan or any of the excipients in SYFOVRE. Systemic hypersensitivity reactions (e.g., anaphylaxis, rash, urticaria) have occurred.
WARNINGS AND PRECAUTIONS
- Endophthalmitis and Retinal Detachments
- Intravitreal injections, including those with SYFOVRE, may be associated with endophthalmitis and retinal detachments. Proper aseptic injection technique must always be used when administering SYFOVRE to minimize the risk of endophthalmitis. Patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment without delay and should be managed appropriately.
- Retinal Vasculitis and/or Retinal Vascular Occlusion
- Retinal vasculitis and/or retinal vascular occlusion, typically in the presence of intraocular inflammation, have been reported with the use of SYFOVRE. Cases may occur with the first dose of SYFOVRE and may result in severe vision loss. Discontinue treatment with SYFOVRE in patients who develop these events. Patients should be instructed to report any change in vision without delay.
- Neovascular AMD
- In clinical trials, use of SYFOVRE was associated with increased rates of neovascular (wet) AMD or choroidal neovascularization (
12% when administered monthly,7% when administered every other month and3% in the control group) by Month 24. Patients receiving SYFOVRE should be monitored for signs of neovascular AMD. In case anti-Vascular Endothelial Growth Factor (anti-VEGF) is required, it should be given separately from SYFOVRE administration.
- In clinical trials, use of SYFOVRE was associated with increased rates of neovascular (wet) AMD or choroidal neovascularization (
- Intraocular Inflammation
- In clinical trials, use of SYFOVRE was associated with episodes of intraocular inflammation including: vitritis, vitreal cells, iridocyclitis, uveitis, anterior chamber cells, iritis, and anterior chamber flare. After inflammation resolves, patients may resume treatment with SYFOVRE.
- Increased Intraocular Pressure
- Acute increase in IOP may occur within minutes of any intravitreal injection, including with SYFOVRE. Perfusion of the optic nerve head should be monitored following the injection and managed as needed.
ADVERSE REACTIONS
- Most common adverse reactions (incidence ≥
5% ) are ocular discomfort, neovascular age-related macular degeneration, vitreous floaters, conjunctival hemorrhage.
Please see full Prescribing Information for more information.
About Apellis
Apellis Pharmaceuticals, Inc. is a global biopharmaceutical company leading the way in complement science to develop life-changing therapies for some of the most challenging diseases patients face. We ushered in the first new class of complement medicine in 15 years and now have two C3-targeting medicines approved to treat four serious diseases. Breakthroughs for patients include the first-ever therapy for geographic atrophy, a leading cause of blindness, and the first treatment for patients 12 and older with C3G or primary IC-MPGN, two severe, rare kidney diseases. We believe we have only begun to unlock the potential of targeting C3 across many serious diseases. For more information, please visit http://apellis.com or follow us on LinkedIn and X.
Apellis Forward-Looking Statement
Statements in this press release about future expectations, plans and prospects, as well as any other statements regarding matters that are not historical facts, may constitute “forward-looking statements” within the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including whether the data analyses reported in this release indicate an apparent positive effect that is greater than the actual positive effect, and other factors discussed in the “Risk Factors” section of Apellis’ Annual Report on Form 10-K with the Securities and Exchange Commission on February 28, 2025 and the risks described in other filings that Apellis may make with the Securities and Exchange Commission. Any forward-looking statements contained in this press release speak only as of the date hereof, and Apellis specifically disclaims any obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.
Media Contact:
Tracy Vineis
media@apellis.com
617.420.4839
Investor Contact:
Eva Stroynowski
ir@apellis.com
617.938.6229
1Rudnicka AR, Jarrar Z, Wormald R, et al. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta analysis. Ophthalmology 2012;119:571–580.
2Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–116.
3Lindblad AS, et al, and AREDS Research Group. Arch Ophthalmol. 2009;127(9):1168-1174.
A graph accompanying this announcement is available at: https://www.globenewswire.com/NewsRoom/AttachmentNg/4b4f80eb-9369-4c1f-8754-5dccbe0b4553








