Asensus Surgical Completes In Vivo Surgeon Lab for LUNA Surgical Robotic System
Surgeons Applaud LUNA's Innovative Capabilities
RESEARCH TRIANGLE PARK, N.C., Jan. 04, 2024 (GLOBE NEWSWIRE) -- Asensus Surgical, Inc. (NYSE American: ASXC), a medical device company that is digitizing the interface between the surgeon and the patient, announced a key development milestone for LUNA™, the Company’s second-generation surgical robotic system.
During the week of December 11, 2023, the Company hosted a Surgeon Lab in Research Triangle Park, North Carolina to conduct an in vivo evaluation of LUNA’s hardware, software and instruments in porcine models. The lab allowed nine participating surgeons to evaluate the system's functionality through thirteen different procedures across gynecology, urology, and general surgery.
Anthony Fernando, President and CEO of Asensus Surgical, provided a detailed update during a recent presentation, highlighting surgeon feedback on the innovative features of LUNA. For a closer look at the Surgeon Lab and insights from the participating surgeons, a video is available on the Company’s website at https://ir.asensus.com/events-and-presentations. The video provides an overview of the system's features, demonstrating its dextrous 5mm TrueWrist™ instruments range, its unique surgeon console and its ergonomic benefits.
"The Surgeon Lab represents a crucial phase in the LUNA development journey," noted Anthony Fernando, President and CEO of Asensus Surgical. "The positive response from the participating surgeons reaffirms our commitment to further developing and delivering a surgical robotic system that not only meets but exceeds expectations. The insights gathered will contribute to the refinement of this late-stage engineering prototype, moving us closer to a finalized product. We will provide further details on LUNA's development and regulatory submission timeline during our upcoming earnings call to be scheduled to report on the fourth quarter and year end 2023.”
"The LUNA system showcased an impressive arm range of motion and instrument dexterity, providing a level of precision that is essential in surgical procedures. The strength and reliability of the 5mm TrueWrist instrument line stood out, offering versatility with different instrument types," mentioned Dr. Amit Trivedi, Chair Department of Surgery, Pascack Valley Medical Center in New Jersey. "The ergonomic design of the LUNA surgeon console and the easy patient access are notable features. I look forward to seeing how the system evolves and the potential positive outcomes it may bring to surgery."
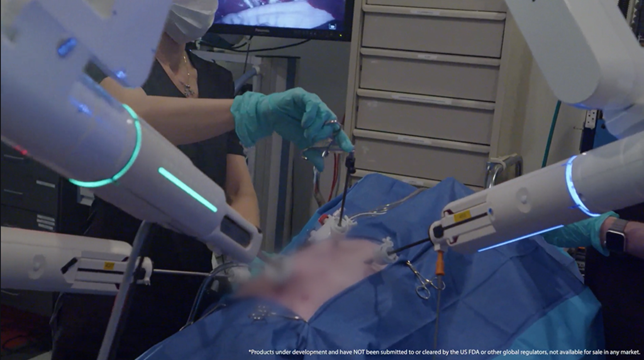
The LUNA Surgical System is Asensus’ next generation digital surgery platform that is poised to revolutionize the way surgery is performed.
LUNA, the Company’s Next Generation Digital Surgery Platform
LUNA is designed to elevate the standards of robotic-assisted surgery. The system integrates carefully crafted hardware and software architecture to prioritize safety, reliability, and performance. LUNA's features include a surgeon console with unconstrained handles, an interactive touchscreen, and an Ultra-HD 3D monitor. Additionally, the system boasts up to four independent robotic manipulator arms for procedural flexibility, alongside a unique instrument drive system supporting various advanced instrumentation options. LUNA is currently under development and has not been submitted to, or cleared by, the US FDA or other global regulators, and is not available for sale in any market.
About Asensus Surgical, Inc.
Asensus Surgical, Inc. is digitizing the interface between the surgeon and patient to pioneer a new era of Performance-Guided Surgery™ by unlocking clinical intelligence for surgeons to enable consistently superior outcomes and a new standard of surgery. Based upon the foundations of Digital Laparoscopy and the Senhance® Surgical System, the Company is developing the LUNA Surgical System, a next generation robotic and instrument system as a foundation of its Digital Surgery solution. These systems will be powered by the Intelligent Surgical Unit™ to increase surgeon control and reduce surgical variability. With the addition of machine vision, Augmented Intelligence, and deep learning capabilities throughout the surgical experience, we intend to holistically address the current clinical, cognitive and economic shortcomings that drive surgical outcomes and value-based healthcare. The Senhance Surgical System is now available for sale in the US, EU, Japan and select other countries. For a complete list of indications for use, visit: www.senhance.com/indications. To learn more about Performance-Guided Surgery, and digital laparoscopy with the Senhance Surgical System visit www.asensus.com.
Follow Asensus
Email Alerts: https://ir.asensus.com/email-alerts
LinkedIn: https://www.linkedin.com/company/asensus-surgical-inc/
Twitter: https://twitter.com/AsensusSurgical
YouTube: https://www.youtube.com/@AsensusSurgical
Vimeo: https://vimeo.com/asxc
TikTok: https://www.tiktok.com/@asensus_surgical
Forward-Looking Statements
This press release includes statements relating to the LUNA Surgical Robotic System development, including the December 2023 surgeon lab and other statements regarding our future plans and goals constitute "forward looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. Such statements are subject to risks and uncertainties that are often difficult to predict, are beyond our control and which may cause results to differ materially from expectations and include whether the LUNA Surgical Robotic System will be successfully developed and receive regulatory clearances on the timeline we anticipate. For a discussion of the risks and uncertainties associated with the Company’s business, please review our filings with the Securities and Exchange Commission (SEC), including our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 2, 2023 and our other filings we make with the SEC. You are cautioned not to place undue reliance on these forward looking statements, which are based on our expectations as of the date of this press release and speak only as of the origination date of this press release. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
INVESTOR CONTACT:
Mark Klausner or Mike Vallie, 443-213-0499
invest@asensus.com
MEDIA CONTACT:
Dan Ventresca
Matter Communications
AsensusPR@matternow.com
617-874-5488
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/37115e8d-9772-4352-a919-c794b2d3aa77






