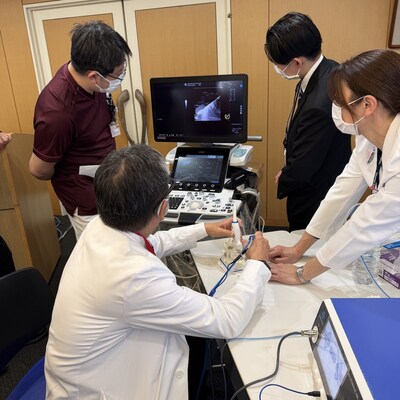
Baird Medical Accelerates Global Commercial Roadmap with Strategic Clinical Education Initiatives in the U.S. and Japan
Rhea-AI Summary
Baird Medical (NASDAQ: BDMD) anunció programas de educación clínica en EE. UU. y Japón para acelerar la adopción global de su tecnología de Ablación por Microondas (MWA) para tumores benignos de tiroides. La empresa lideró una capacitación avanzada en la Universidad de Columbia y un taller en la Showa Medical University para estandarizar las técnicas de tiroidología intervencional y ampliar las opciones para pacientes no quirúrgicos.
Positive
- None.
Negative
- None.
News Market Reaction
On the day this news was published, BDMD gained 9.73%, reflecting a notable positive market reaction. Argus tracked a peak move of +3.1% during that session. Our momentum scanner triggered 3 alerts that day, indicating moderate trading interest and price volatility. This price movement added approximately $3M to the company's valuation, bringing the market cap to $37M at that time.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
BDMD was up 1.74% with no peers in the real-time momentum scanner. Broader medical-device peers showed mixed moves (e.g., ICAD and ICCM up, APYX and XTNT down), pointing to a stock-specific reaction rather than a coordinated sector move.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Jan 28 | Clinical education event | Positive | +6.9% | Gynecology microwave ablation masterclass to train physicians across Asia. |
| Jan 26 | Strategic partnership | Positive | +0.0% | Partnership to use Hainan Free Trade Port as hub for global commercialization. |
| Jan 20 | Investor conference | Positive | -0.9% | JP Morgan Healthcare Conference meetings to discuss strategy and MWA adoption. |
| Jan 07 | Regulatory approvals | Positive | +3.9% | Product registrations in Pakistan and Vietnam enabling new market entries. |
| Dec 17 | Manufacturing expansion | Positive | +2.2% | Establishment of U.S. manufacturing base with MPS Medical for global supply. |
Recent news around global expansion, manufacturing, and education has more often been followed by positive one-day price reactions than negative ones.
Over the past few months, BDMD has repeatedly highlighted its global commercialization push. It established a U.S. manufacturing base on Dec. 17, 2025, secured regulatory approvals in Pakistan and Vietnam on Jan. 7, 2026, and engaged investors at the 2026 J.P. Morgan Healthcare Conference on Jan. 20. Subsequent news on partnerships and physician-education masterclasses reinforced this strategy. Today’s U.S. and Japan clinical education initiatives continue that theme of expanding minimally invasive MWA adoption and physician training across key markets.
Market Pulse Summary
The stock moved +9.7% in the session following this news. A strong positive reaction aligns with BDMD’s pattern of favorable responses to global expansion and education news, where several recent announcements saw positive one-day moves. However, the stock traded near its 52-week low of 0.901 and well below its 200-day MA of 2.64, highlighting prior weakness. The sizable registered resale overhang of 34,415,562 shares and broader regulatory risks noted in filings could still weigh on longer-term sentiment.
Key Terms
microwave ablation medical
interventional thyroidology medical
benign thyroid tumors medical
public warrants financial
hfCAA regulatory
AI-generated analysis. Not financial advice.
In
Simultaneously, Baird Medical solidified its presence in
About Baird Medical
Baird Medical is a forward-thinking medical device company specializing in minimally invasive diagnostics and treatment. It is dedicated to the research and development of surgical robotic systems and innovative minimally invasive surgical instruments. Our mission is to enhance patient outcomes through precision technology and advanced diagnostic solutions. The company will foster strategic collaborations with leading academic institutions. Our vision extends beyond surgical assistance, aiming to develop intelligent systems that proactively guide diagnostic decisions and preventive healthcare strategies. As an FDA 510(k)-certified medical device company, Baird Medical's solutions have been used in over 30 prestigious hospitals and clinics across
Forward-Looking Statements
This press release includes certain statements that are not historical facts but are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements generally relate to future events or Baird Medical's future financial or operating performance. In some cases, you can identify forward-looking statements by terminology such as "may", "could", "should", "expect", "intend", "might", "will", "estimate", "anticipate", "believe", "budget", "forecast", "intend", "plan", "potential", "predict", or "continue", or the negatives of these terms or variations of them or similar terminology. Forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Baird Medical and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. You should not place undue reliance on forward-looking statements in this press release, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein. Baird Medical does not undertake any duty to update these forward-looking statements.
Actual results may vary materially from those expressed or implied by forward-looking statements based on a number of factors, including, without limitation: (1) the risk that Baird Medical may not be successful in expanding its business in China or the
The foregoing list of factors is not exclusive. Additional information concerning certain of these, and other risk factors is contained in ExcelFin's most recent filings with the SEC and in the Registration Statement described above filed by Baird Medical in connection with its business combination with ExcelFin. All subsequent written and oral forward-looking statements concerning Baird Medical, the business combination described herein or other matters attributable to Baird Medical or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements above. Readers are cautioned not to place undue reliance upon any forward-looking statements, which speak only as of the date made. Baird Medical expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in their expectations with respect thereto or any change in events, conditions or circumstances on which any statement is based.
Contact:
Eric Huang, PR Liaison
Baird Medical Investment Holdings Ltd.
Phone: +1 (888) 508-6228
Email: ir@bairdmed.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/baird-medical-accelerates-global-commercial-roadmap-with-strategic-clinical-education-initiatives-in-the-us-and-japan-302682296.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/baird-medical-accelerates-global-commercial-roadmap-with-strategic-clinical-education-initiatives-in-the-us-and-japan-302682296.html
SOURCE BDMD