Biomea Fusion’s BMF-500 Selected for Poster Presentation at EHA 2025 Highlighting Phase I Data in Relapsed/Refractory Acute Leukemia
Rhea-AI Summary
- 24 patients enrolled, with 15 having FLT3 mutations
- 81.8% of evaluable patients showed clinical activity
- 77.8% demonstrated decreased bone marrow blasts
- Median overall survival of 3.48 months vs. historical 2.1 months
- Well-tolerated safety profile with no treatment-related discontinuations
Positive
- 81.8% of evaluable patients showed clinical activity with decreased bone marrow blasts
- Median overall survival exceeded historical benchmarks (3.48 months vs 2.1 months)
- BMF-500 demonstrated good safety profile with no treatment-related discontinuations
- Company actively pursuing strategic partnerships for BMF-500 development
Negative
- Company shifting away from oncology focus to metabolic diseases
- Only 11 out of 24 enrolled patients were evaluable for efficacy
- Only one patient achieved CRi (Complete Remission with incomplete hematologic recovery)
News Market Reaction 1 Alert
On the day this news was published, BMEA declined 2.05%, reflecting a moderate negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
REDWOOD CITY, Calif., May 14, 2025 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. (“Biomea”) (Nasdaq: BMEA), a clinical-stage diabetes and obesity medicines company, today announced that preliminary clinical data from the Phase I COVALENT-103 trial of BMF-500 in adults with acute leukemia (AL) were selected for a poster presentation at the European Hematology Association (EHA) 2025 Congress, taking place June 12–15 in Milan, Italy.
The presentation will highlight emerging safety, pharmacokinetics/pharmacodynamics (PK/PD), and clinical activity of BMF-500, a covalent FLT3 inhibitor, in patients with relapsed or refractory (R/R) AL, including those with FLT3 mutations (FLT3m) who have previously received FLT3 inhibitors such as gilteritinib (gilt).
“While we have strategically shifted our internal focus to metabolic disease, the preliminary results from the COVALENT-103 study underscore the strong potential of BMF-500 in relapsed or refractory acute leukemia. Despite having received and failed multiple prior lines of therapy, the majority of treated patients experienced reductions in bone marrow blasts. Early signs of overall survival already exceed historical benchmarks, even at non-optimized dose levels,” said Mick Hitchcock, Ph.D., Interim Chief Executive Officer of Biomea Fusion. “We are actively advancing partnership discussions for this very selective and active covalent binding molecule which was developed in-house for patients with very limited treatment options.”
Abstract and Poster Presentation Details
- Date/Time: Saturday, June 14 (18:30-19:30 CEST)
- Title: Covalent FLT3 Inhibitor BMF-500 in Relapsed or Refractory (R/R) Acute Leukemia (AL): Preliminary Phase 1 Data from the COVALENT-103 Study (NCT05918692)
- Poster Number: PS1520
- Presenter: Farhad Ravandi-Kashani, M.D., University of Texas MD Anderson Cancer Center
Background
R/R FLT3m AL post-failure with gilteritinib (gilt) has a poor prognosis. BMF-500 is a covalent FLT3 inhibitor, potent against ITD, TKD, and resistance mutations like the gatekeeper F691. BMF-500 lacks cKIT inhibition, exhibits cytotoxicity even after washout, and elicits improved survival in FLT3m AML xenografts.
Aims
Here we update the ongoing COVALENT-103 study, an open-label Phase I study evaluating escalating doses of BMF-500 in R/R AML with or without FLT3m.
Methods
Eligible pts are adults with R/R AL ineligible for standard of care. Pts with FLT3m AL must have failed gilt in the R/R setting. Up to
Results
As of 03Feb25, 24 R/R AL pts enrolled; 4 remain on treatment. Baseline features: 8 (
BMF-500 was well tolerated with no DLTs or discontinuations due to treatment-related toxicities, and no related QTc prolongation or cytopenias. Twenty-three pts comprised the safety population. Common TEAEs (>
The highest levels cleared are 100 mg BID (Arm A /DL2) and 50 mg BID (Arm B /DL2), with 3 of 3 (
Based on exposures surpassing the preclinical target AUC, the study pivoted from single-patient cohorts to 3+3 at 100 mg BID (Arm A) and 25 mg BID (Arm B). Exposures were comparable at these two dose levels and Plasma Inhibitory Assay showed near complete FLT3 inhibition at steady state. PK/PD showed an EC90 of 500 ng/mL, with most pts at 100 mg BID and 25 mg BID surpassing it. BMF-500 and its metabolites had similar concentrations in BM and plasma.
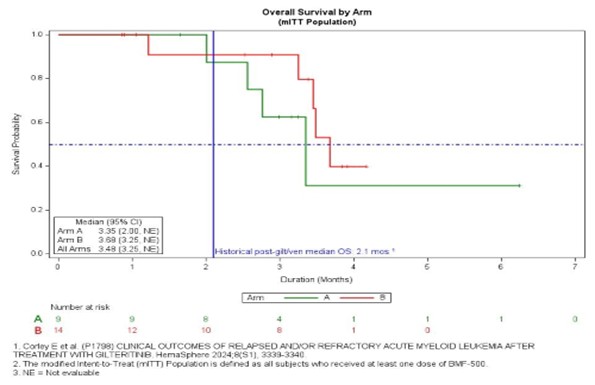
Summary/Conclusion
BMF-500 was well-tolerated. The majority of efficacy-evaluable pts showed reduced BM blasts, with 1 pt achieving CRi. mOS of the efficacy-evaluable FLT3m pts has not yet been reached. Enrollment is ongoing
to identify the OBD/RP2D.
Following completion of the dose escalation phase in relapsed/refractory acute leukemia patients with FLT3 mutations, Biomea plans to conclude its internal development of BMF-500 in oncology and is actively exploring strategic partnerships to advance the program.
About COVALENT-103
COVALENT-103 is a multicenter, open-label, non-randomized trial seeking to evaluate the safety and efficacy of BMF-500, a twice daily oral treatment, in adult patients with relapsed or refractory acute leukemia with FMS-like tyrosine kinase 3 (FLT3) wild-type and FLT3 mutations. The Phase I COVALENT-103 study aims to evaluate the safety and tolerability of BMF-500, determine the optimal biologic dose and recommended Phase II dose. Additional information about the Phase I clinical trial of BMF-500 can be found at ClinicalTrials.gov using the identifier, NCT05918692.
About BMF-500
BMF-500 is an investigational, orally bioavailable, covalent small molecule inhibitor of FLT3, discovered and developed in-house at Biomea using the company’s proprietary FUSION™ System. Designed to be highly potent and selective, BMF-500 has demonstrated encouraging potential in extensive preclinical studies. Its kinase inhibitory profile indicates strong target selectivity, which may translate to a reduced risk of off-target effects.
About Biomea Fusion
Biomea Fusion is a clinical-stage diabetes and obesity medicines company focused on the development of its oral small molecules, icovamenib and BMF-650, both designed to significantly improve the lives of patients with diabetes, obesity, and metabolic diseases. We aim to cure.
Visit us at biomeafusion.com and follow us on LinkedIn, X, and Facebook.
Forward-Looking Statements
Statements we make in this press release may include statements which are not historical facts and are considered forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These statements may be identified by words such as “aims,” “anticipates,” “believes,” “could,” “estimates,” “expects,” “forecasts,” “goal,” “intends,” “may,” “plans,” “possible,” “potential,” “seeks,” “will,” and variations of these words or similar expressions that are intended to identify forward-looking statements. Any such statements in this press release that are not statements of historical fact, including statements regarding the clinical and therapeutic potential of our product candidates and development programs, including BMF-500, the potential of BMF-500 as a treatment for patients with FLT3m R/R AL, our research, development, partnership and regulatory plans, and the timing of such events may be deemed to be forward-looking statements. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Exchange Act and are making this statement for purposes of complying with those safe harbor provisions. Any forward-looking statements in this press release are based on our current expectations, estimates and projections only as of the date of this release and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements, including the risk that preliminary or interim results of preclinical studies or clinical trials may not be predictive of future or final results in connection with future clinical trials and the risk that we may encounter delays in preclinical or clinical development, patient enrollment and in the initiation, conduct and completion of our ongoing and planned clinical trials and other research and development activities. These risks concerning Biomea’s business and operations are described in additional detail in its periodic filings with the U.S. Securities and Exchange Commission (SEC), including its most recent periodic report filed with the SEC and subsequent filings thereafter. Biomea Fusion explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Contact:
Meichiel Jennifer Weiss
Sr. Director, Investor Relations and Corporate Development
IR@biomeafusion.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/30df3ec6-a213-439f-8d74-cc730ef0127c








