Benitec Biopharma Provides Positive Long-Term Clinical Study Results for BB-301 Phase 1b/2a Clinical Trial Demonstrating Robust Efficacy and Continued Durability of Response
Rhea-AI Summary
Benitec Biopharma (NASDAQ: BNTC) reported positive long-term results from the BB-301 Phase 1b/2a study in oculopharyngeal muscular dystrophy (OPMD). The first patient in Cohort 1 completed a 24-month follow-up and showed durable, deepening improvements in swallowing function versus pre-treatment and 12-month assessments.
Key objective measures improved and/or deepened at 24 months: PhAMPC +27% (maintained), NRRSv -60%, TPR -39%, and patient-reported SSQ -78%. All 4 Cohort 1 completers met prespecified responder criteria at month 12.
Positive
- PhAMPC +27% maintained at 24 months for Patient 1
- NRRSv -60% reduction in vallecular residue for Patient 1 at 24 months
- TPR -39% reduction in total pharyngeal residue for Patient 1 at 24 months
- SSQ -78% patient-reported reduction in dysphagic symptom burden for Patient 1 at 24 months
- All 4 Cohort 1 completers were formal Responders at 12 months
Negative
- Efficacy durability at 24 months is reported for only one patient to date
- Analysis is limited to 4 Cohort 1 completers for the 12-month responder assessment
Key Figures
Market Reality Check
Peers on Argus
Pre-news, BNTC fell 2.79% while peers were mixed: AUTL -6.7%, LRMR -2.06%, DMAC -2.63%, GALT -0.32%, and ADCT +1.1%, suggesting stock-specific dynamics rather than a unified biotech move.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Nov 14 | Earnings & update | Positive | +2.8% | Q1 2026 results, BB-301 progress, and ~<b>$100M</b> equity financing. |
| Nov 05 | Equity offering | Negative | +0.5% | Proposed underwritten and registered direct offerings under Form S-3. |
| Nov 03 | Clinical results | Positive | -3.1% | Positive BB-301 interim data and FDA Fast Track designation. |
| Nov 03 | Board appointment | Positive | -3.1% | Appointment of experienced biotech leader Sharon Mates to board. |
| Nov 02 | Clinical update notice | Neutral | -3.1% | Announcement of upcoming BB-301 Phase 1b/2a clinical update webcast. |
Recent clinically focused and financing news often saw price moves that diverged from the seemingly positive tone, especially around BB-301 clinical updates.
Over the last few months, Benitec has centered its story on BB-301 for OPMD. On Nov 3, 2025, positive Phase 1b/2a interim data and FDA Fast Track coincided with a -3.06% move, following a clinical update pre-announcement that also saw a -3.06% reaction. Earlier in Feb–Mar 2025, interim clinical data and conference visibility for BB-301 produced smaller positive moves up to 15.98%. Earnings on Nov 14, 2025 combined clinical progress with strengthened cash, leading to a 2.81% gain. Today’s long-term durability update extends that same BB-301 clinical narrative.
Regulatory & Risk Context
An effective Form S-3 shelf dated 2025-09-22 allows Benitec to offer various securities, including up to $94,541,030 of securities carried over from a November 2024 shelf, supporting future capital raises alongside prior usage via multiple 424B5 offerings.
Market Pulse Summary
This announcement reinforces BB-301’s profile with 12–24 month durability data, including up to 78% improvement in dysphagic symptom scores and consistent gains across VFSS-based swallowing metrics for Patient 1, alongside 4/4 responders among early Cohort 1 completers. Prior clinical news for this program has meaningfully moved the stock, with an average shift of 5.75%. Investors may watch upcoming FDA interactions in 2026, further Cohort 2 data, and any additional capital raises under the remaining $94,541,030 shelf capacity.
Key Terms
videofluoroscopic swallowing studies (vfss) medical
sydney swallow questionnaire (ssq) medical
pharyngeal area at maximum constriction (phampc) medical
normalized residue ratio scale-valleculae (nrrsv) medical
total pharyngeal residue (tpr) medical
responder analysis technical
u.s. food and drug administration (fda) regulatory
AI-generated analysis. Not financial advice.
• Patient 1 of Cohort 1 has now completed the 24-month follow-up timepoint, and at month-24 post-treatment Patient 1 continued to demonstrate the powerful disease-modifying effects of BB-301, with deepening improvements in post-swallow residue and total dysphagic symptom burden as compared to the 12-month follow-up timepoint
• Patient 4 of Cohort 1 continued to experience strong response to BB-301 at the 12-month follow-up timepoint
• The first 4 patients enrolled into Cohort 1 have completed the 12-month statistical follow-up period, and all 4 Completers were formal Responders to BB-301 at the month-12 follow-up timepoint demonstrating durable response to BB-301
HAYWARD, Calif., Jan. 11, 2026 (GLOBE NEWSWIRE) -- Benitec Biopharma Inc. (NASDAQ: BNTC) (“Benitec” or “Company”), a clinical-stage, gene therapy-focused, biotechnology company developing novel genetic medicines based on its proprietary “Silence and Replace” DNA-directed RNA interference (“ddRNAi”) platform, today announced that the first patient treated in Cohort 1 of the BB-301 Phase 1b/2a clinical study (NCT06185673) evaluating BB-301 for the treatment of dysphagia in oculopharyngeal muscular dystrophy (OPMD) has completed the 24-month post-treatment assessment. At the 24-month follow-up timepoint, Patient 1 continued to demonstrate robust, disease-modifying outcomes. At the 24-month follow-up timepoint, Patient 1 demonstrated deepening improvements in post-swallow pharyngeal residue as compared to the final pre-treatment timepoint and the 12-month post-treatment follow-up timepoint as assessed by x-ray-based swallowing studies. Additionally, Patient 1 experienced deepening improvements in total dysphagic symptom burden as assessed by the Sydney Swallow Questionnaire (SSQ). The first 4 patients in Cohort 1 have now completed the 12-month statistical follow-up period for the Phase 1b/2a study, and all 4 Completers continued to demonstrate durable response to BB-301. All 4 Cohort 1 Completers met the pre-specified statistical criteria for response to BB-301 defined by Benitec which require improvement across 2 or more of the 5 categories of assessment comprising the Responder Analysis1.
“Progressive dysphagia is the most severe, life-threatening complication of OPMD, and we are extremely excited to observe safe, durable, disease-modifying outcomes for the patients treated with BB-301,” said Jerel A. Banks, M.D., Ph.D., Executive Chairman and Chief Executive Officer of Benitec Biopharma Inc. “Durable improvements in the dysphagic symptom burden can have a profound impact on the lives of patients living with OPMD, and we remain singularly focused on advancing BB-301 through development to improve the lives of all OPMD patients. We look forward to engaging with the U.S. Food and Drug Administration (FDA) in mid-2026 to confirm the BB-301 pivotal study design and continuing to present interim clinical results at future medical conferences. We extend our deepest gratitude to the patients and families participating in the clinical study and to the investigators and clinical teams for their dedication to advancing new treatment options.”
Updated Interim Clinical Study Results
24-Month Post-Treatment Follow-Up for Patient 1 of Cohort 1
At the 24-month post-BB-301 treatment follow-up timepoint, Patient 1 of Cohort 1 continued to demonstrate robust, disease-modifying outcomes. At the 24-month follow-up timepoint, Patient 1 demonstrated deepening improvements in post-swallow pharyngeal residue as compared to the final pre-treatment timepoint and as compared to the 12-month post-treatment follow-up timepoint as assessed by videofluoroscopic swallowing studies (VFSS). Patient 1 also experienced deepening improvements in total dysphagic symptom burden as assessed by the Sydney Swallow Questionnaire (SSQ).
- Pharyngeal Area at Maximum Constriction (“PhAMPC”), as assessed by VFSS, represents the functional capacity of the pharyngeal constrictor muscles during the swallowing cycle
- At the 24-month post-treatment timepoint, Patient 1 demonstrated durable functional improvement of pharyngeal constrictor-mediated throat closure, as the 12-month post-treatment improvements were perfectly maintained at the 24-month post-treatment timepoint
- As compared to the final pre-treatment visit, Patient 1 demonstrated a
27% improvement in PhAMPC (throat closure) at the 12-month post-treatment timepoint (27% improvement in throat closure at the peak of swallowing as compared to the final pre-treatment assessment), and the27% improvement in PhAMPC was maintained at the 24-month post treatment timepoint (27% improvement in throat closure at the peak of swallowing as compared to the final pre-treatment assessment), indicating durable improvement in pharyngeal muscle function during swallowing
- Normalized Residue Ratio Scale-Valleculae (NRRSv), as assessed by VFSS, represents the quantity of food and liquid material (residue) remaining in the vallecular region of the throat upon completion of a swallow (post-swallow residue)
- Elevated levels of post-swallow residue in the vallecular region of the throat have been shown to correlate with increased risk of aspiration events
- At the 24-month post-treatment timepoint, the throat-emptying ability of Patient 1 continued to significantly improve for liquids and solid food
- As compared to the final pre-treatment visit, Patient 1 demonstrated a
35% improvement in NRRSv at the 12-month post-treatment timepoint (35% reduction in post-swallow residue in the vallecular region as compared to the final pre-treatment assessment), and at the 24-month post-treatment timepoint Patient 1 demonstrated a60% improvement in NRRSv (60% reduction in post-swallow residue in the vallecular region as compared to the final pre-treatment assessment) exemplifying a deepening of the improvement in swallowing efficiency over time following the administration of BB-301
- Total Pharyngeal Residue (TPR), as assessed by VFSS, represents the quantity of food and liquid material (residue) remaining in the throat upon completion of a swallow (post-swallow residue)
- At the 24-month post-treatment timepoint, the throat-emptying ability of Patient 1 continued to significantly improve for liquids and solid food
- As compared to the final pre-treatment visit, Patient 1 demonstrated a
32% improvement in TPR at the 12-month post-treatment timepoint (32% reduction in post-swallow residue as compared to the final pre-treatment assessment), and at the 24-month post-treatment timepoint Patient 1 demonstrated a39% improvement in TPR (39% reduction in post-swallow residue as compared to the final pre-treatment assessment) exemplifying a deepening of the improvement in swallowing efficiency over time following the administration of BB-301
- Sydney Swallow Questionnaire, a validated 17-item patient-reported outcome instrument, represents the total dysphagic symptom burden experienced by a patient
- At the 24-month post-treatment timepoint, the total dysphagic symptom burden continued to significantly decline
- As compared to the final pre-treatment visit, Patient 1 experienced a
64% improvement in SSQ total score at the 12-month post-treatment timepoint (64% reduction in total dysphagic symptom burden as compared to the final pre-treatment assessment), and at the 24-month post-treatment timepoint Patient 1 experienced a78% improvement in SSQ total score (78% reduction in total dysphagic symptom burden as compared to the final pre-treatment assessment) exemplifying a deepening of the improvement in total dysphagic symptom burden over time following the administration of BB-301
Analysis of Study Completers (12-Month Post-Treatment Follow-Up)
A Responder Analysis was developed to facilitate standardized evaluation of BB-301 efficacy for each patient. The Responder Analysis consists of multiple discrete response categories that collectively assess the dysphagic symptom burden in patients with OPMD.
- These response categories include:
- Patient-Reported Outcome: Patient-reported oropharyngeal dysphagia as assessed by the Sydney Swallow Questionnaire (SSQ) total score
- Videofluoroscopic Swallowing Study (VFSS) Assessments:
- Pharyngeal constrictor muscle function as estimated by the Pharyngeal Area at Maximum Constriction (PhAMPC)
- Swallowing efficiency as measured by NRRSv and Total Pharyngeal Residue %(C2-4)2
- Frequency of pathologic sequential swallows (SEQ)
- Functional Swallowing Capacity: Cold-Water Timed Drinking Test (CWDT)
- Following completion of the 12-month post-treatment follow-up timepoint, each discrete response category is evaluated for each study Completer using prespecified statistical criteria
- Results of the statistical characterization of each response category are combined into a single scoring framework that facilitates the overall assessment of clinical benefit achieved by each patient following treatment with BB-301
- A total Score of 5 is possible
- Responder status for each patient will be assigned based on the achievement of statistical criteria for at least 2 out of 5 discrete response categories (≥
40% )
- Patient-Reported Outcome: Patient-reported oropharyngeal dysphagia as assessed by the Sydney Swallow Questionnaire (SSQ) total score
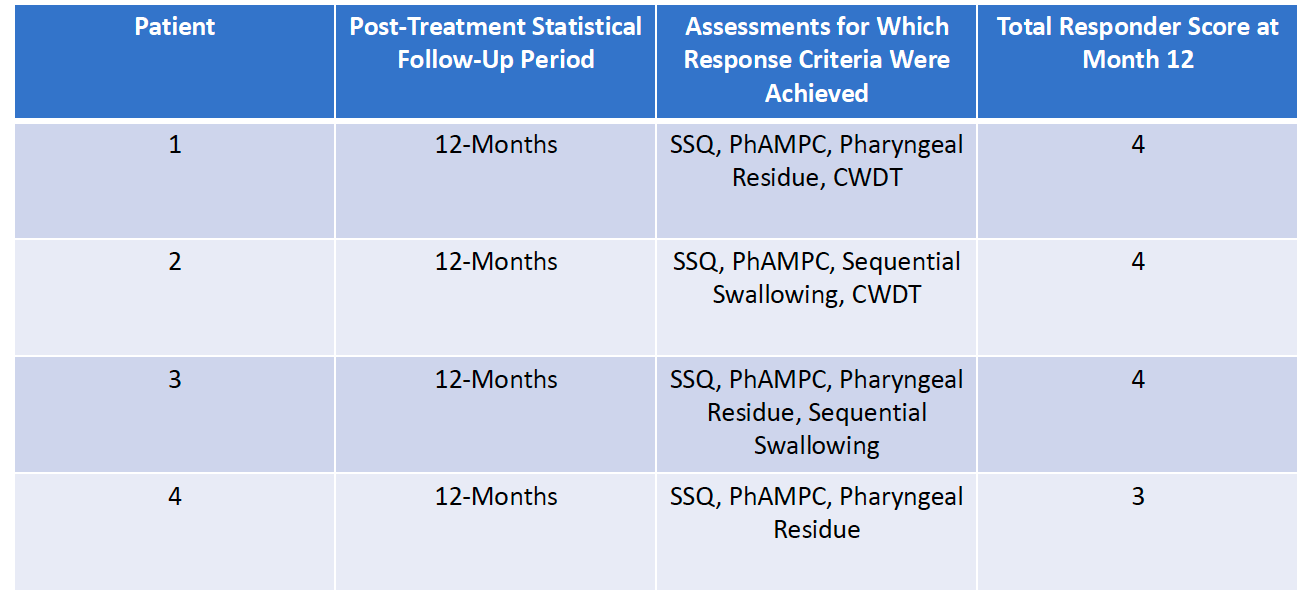
Responder Analysis for Study Completers: Patients 1-to-4 of Cohort 1
All 4 Cohort 1 Completers were formal Responders to BB-301, demonstrating durable response to BB-301 at the conclusion of the 12-month statistical follow-up period.
About BB-301
BB-301 is a novel, modified AAV9 capsid expressing a unique, single bifunctional construct promoting co-expression of both codon-optimized Poly-A Binding Protein Nuclear-1 (PABPN1) and two small inhibitory RNAs (siRNAs) against mutant PABPN1 (the causative gene for OPMD). The two siRNAs are modeled into microRNA backbones to silence expression of faulty mutant PABPN1, while allowing expression of the codon-optimized PABPN1 to replace the mutant with a functional version of the protein. We believe the silence and replace mechanism of BB-301 is uniquely positioned for the treatment of OPMD by halting mutant expression while providing a functional replacement protein. BB-301 has received Orphan Drug Designation from the EMA and Orphan Drug and Fast Track Designations from the FDA.
About Benitec Biopharma, Inc.
Benitec Biopharma Inc. (“Benitec” or the “Company”) is a clinical-stage biotechnology company focused on the advancement of novel genetic medicines with headquarters in Hayward, California. The proprietary “Silence and Replace” DNA-directed RNA interference platform combines RNA interference, or RNAi, with gene therapy to create medicines that simultaneously facilitate sustained silencing of disease-causing genes and concomitant delivery of wildtype replacement genes following a single administration of the therapeutic construct. The Company is developing Silence and Replace-based therapeutics for chronic and life-threatening human conditions including Oculopharyngeal Muscular Dystrophy (OPMD). A comprehensive overview of the Company can be found on Benitec’s website at www.benitec.com.
Forward Looking Statements
Except for the historical information set forth herein, the matters set forth in this press release include forward-looking statements, including statements regarding Benitec’s plans to develop and commercialize its product candidates, the timing of the completion of pre-clinical and clinical trials, the timing of the availability of data from our clinical trials, the timing and sufficiency of patient enrollment and dosing in clinical trials, the timing of expected regulatory filings, and the clinical utility and potential attributes and benefits of ddRNAi and Benitec’s product candidates, and other forward-looking statements.
These forward-looking statements are based on the Company’s current expectations and subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: the success of our plans to develop and potentially commercialize our product candidates; the timing of the completion of preclinical studies and clinical trials; the timing and sufficiency of patient enrollment and dosing in any future clinical trials; the timing of the availability of data from our clinical trials; the timing and outcome of regulatory filings and approvals; the development of novel AAV vectors; our potential future out-licenses and collaborations; the plans of licensees of our technology; the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, including the potential duration of treatment effects and the potential for a “one shot” cure; our intellectual property position and the duration of our patent portfolio; expenses, ongoing losses, future revenue, capital needs and needs for additional financing, and our ability to access additional financing given market conditions and other factors; the length of time over which we expect our cash and cash equivalents to be sufficient to execute on our business plan; unanticipated delays; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of subjects in clinical trials; determinations made by the FDA and other governmental authorities and other regulatory developments; the Company’s ability to protect and enforce its patents and other intellectual property rights; the Company’s dependence on its relationships with its collaboration partners and other third parties; the efficacy or safety of the Company’s products and the products of the Company’s collaboration partners; the acceptance of the Company’s products and the products of the Company’s collaboration partners in the marketplace; market competition; sales, marketing, manufacturing and distribution requirements; greater than expected expenses; expenses relating to litigation or strategic activities; the impact of, and our ability to remediate, the identified material weakness in our internal controls over financial reporting; the impact of local, regional, and national and international economic conditions and events; and other risks detailed from time to time in the Company’s reports filed with the Securities and Exchange Commission. The Company disclaims any intent or obligation to update these forward-looking statements.
Investor Relations Contact:
Irina Koffler
LifeSci Advisors, LLC
(917) 734-7387
ikoffler@lifesciadvisors.com
1 (Final+Webcast+Slides)BENITEC,+2025NOV01.pdf
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f653cced-806c-4f5a-a3ea-991da27438a7









