Dateline Returns Wide Gold Intercepts at Colosseum
Rhea-AI Summary
Coya Therapeutics (NASDAQ: COYA) launched the ALSTARS Trial, a Phase 2, randomized, multi-center, double-blind, placebo-controlled 24-week study of COYA 302 in patients with amyotrophic lateral sclerosis (ALS) (ClinicalTrials.gov: NCT 07161999).
The study will enroll 120 participants across approximately 25 centers in the United States and Canada. Participants completing the 24-week placebo-controlled phase may join a 24-week blinded extension during which all participants will receive COYA 302 at one of two prespecified doses. Study details and eligibility are available on the trial registry. The trial will be presented on September 29, 2025 during a NEALS educational webinar.
Positive
- Phase 2 randomized trial launched with 120 participants
- 24-week placebo-controlled period plus 24-week blinded extension
- Multi-center study at ~25 sites in US and Canada
Negative
- No efficacy data yet; trial has just launched
- Sample size 120 may limit subgroup/statistical power
SAN BERNARDINO, CALIFORNIA / ACCESS Newswire / November 18, 2025 / Dateline Resources Limited (ASX:DTR)(OTCQB:DTREF)(FSE:YE1) is pleased to announce that recent drilling at its
Highlights
Strong gold intercepts from infill drillingconfirm the continuity of mineralisation at depth:
RC25-020 returned 85.34m @ 1.33 g/t Au (beneath the existing pit) and,
CM25-19 returned 61.26m @ 1.18 g/t Au (partial result; remaining assays pending),
Assays received in line with expectations and demonstrate consistent grades over broad intervals, supporting the geological model.
Expanded M&I mineral resource to strengthen Feasibility Study: A larger high-confidence (M&I) resource base will underpin the Bankable Feasibility Study (BFS), enhancing mine design and economic robustness.
The standout drilling intercepts include 85.34 metres at 1.33 g/t Aufrom RC25-020, a reverse-circulation hole drilled beneath the current pit floor, and 61.26 metres at 1.18 g/t Aufrom CM25-19 (a diamond core hole for which assays are still pending beyond this interval). Hole RC25-020's long intercept of consistent gold grades indicates a continuation of the gold mineralisation below the mined pit, extending the known mineralised envelope at depth. Hole CM25-19, an infill hole targeting a previously sparsely drilled zone, has already delivered a substantial gold interval in its upper section.
The breadth and grade of these intersections are comparable to the deposit's average mineral resource grade, reinforcing confidence that the geological model is accurate and that gold mineralisation persists as predicted.
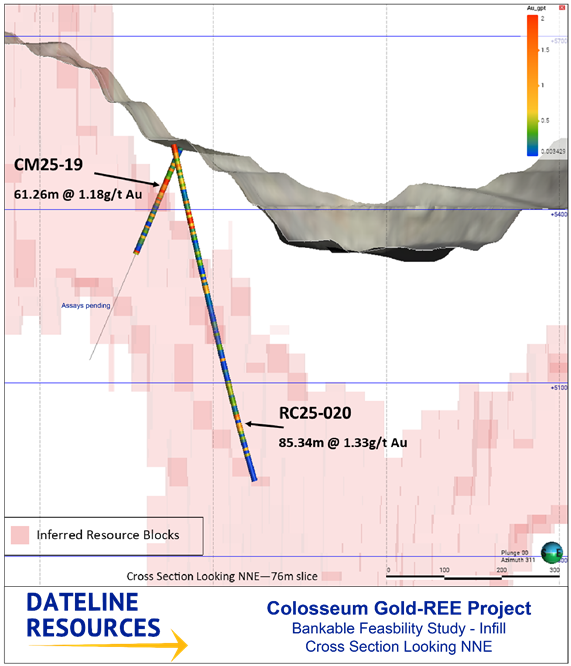
Figure 1: Cross-section of the Colosseum deposit illustrating the existing open pit outline and gold mineralized breccia pipe (blue modelled mineral resource zones). The approximate positions of key new drill intercepts (e.g., RC25-020 and CM25-19) are highlighted below the current pit. These results demonstrate the extension of gold mineralization at depth beneath the pit, supporting the planned upgrade of Inferred resources to higher-confidence categories.
The current JORC-2012 Mineral Resource for the Colosseum Gold deposit stands at 27.1 million tonnes @ 1.26 g/t Au for 1.1 million ounces of gold. Approximately
In summary, the latest drill results from Colosseum are in line with expectations and confirm the robustness of the geological model. They substantiate the Company's approach to upgrade the mineral resource classification, thereby increasing the proportion of gold ounces in the Measured and Indicated categories. This will provide a stronger foundation for mine development plans and improve the quality of the BFS.
Dateline will continue to update shareholders as additional assay results are received and as the JORC mineral resource update process progresses. The Company is confident that the ongoing drilling will add tangible value to the Colosseum Gold-REE Project by enhancing the mineral resource base and de-risking the path to production.
This press release has been authorized for release by the Board of Dateline Resources Limited.
For more information, please contact:
Stephen Baghdadi
Managing Director
+61 2 9375 2353
Andrew Rowell
Corporate & Investor Relations Manager
+61 400 466 226
a.rowell@dtraux.com
www.datelineresources.com.au
Follow Dateline on socials:
X - @Dateline_DTR
Truth Social - @dateline_resources
LinkedIn - dateline-resources
About Dateline Resources Limited
Dateline Resources Limited (ASX:DTR)(OTCQB:DTREF)(FSE:YE1.F) is an Australian company focused on mining and exploration in North America. The Company owns
The Colosseum Gold Mine is located in the Walker Lane Trend in East San Bernardino County, California. On 6 June 2024, the Company announced to the ASX that the Colosseum Gold mine has a JORC-2012 compliant Mineral Resource estimate of 27.1Mt @ 1.26g/t Au for 1.1Moz. Of the total Mineral Resource, 455koz @ 1.47/t Au (
On 23 May 2025, Dateline announced that updated economics for the Colosseum Gold Project generated an NPV6.5 of US
The Colosseum is located less than 10km north of the Mountain Pass Rare Earth mine. Planning has commenced on drill testing the REE potential at Colosseum.
Dateline has also acquired the high-grade Argos Strontium Project, also located in San Bernadino County, California. Argos is reportedly the largest strontium deposit in the U.S. with previous celestite production grading
Forward-Looking Statements
This announcement may contain "forward-looking statements" concerning Dateline Resources that are subject to risks and uncertainties. Generally, the words "will", "may", "should", "continue", "believes", "expects", "intends", "anticipates" or similar expressions identify forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause actual results to differ materially from those expressed in the forward-looking statements. Many of these risks and uncertainties relate to factors that are beyond Dateline Resources' ability to control or estimate precisely, such as future market conditions, changes in regulatory environment and the behavior of other market participants. Dateline Resources cannot give any assurance that such forward-looking statements will prove to have been correct. The reader is cautioned not to place undue reliance on these forward-looking statements. Dateline Resources assumes no obligation and does not undertake any obligation to update or revise publicly any of the forward-looking statements set out herein, whether as a result of new information, future events or otherwise, except to the extent legally required.
Competent Person Statement
Sample preparation and any exploration information in this announcement is based upon work reviewed by Mr Greg Hall who is a Chartered Professional of the Australasian Institute of Mining and Metallurgy (CP-IMM). Mr Hall has sufficient experience that is relevant to the style of mineralization and type of deposit under consideration and to the activity which he is undertaking to qualify as a Competent Person as defined in the 2012 Edition of the "Australasian Code for Reporting Exploration Results, Mineral Resources and Ore Reserves" (JORC Code). Mr Hall is a Non-Executive Director of Dateline Resources Limited and consents to the inclusion in the report of the matters based on this information in the form and context in which it appears.
Company Confirmations
The Company confirms it is not aware of any new information or data that materially affects the information included in the announcements dated 23 October 2024 with regard to the Colosseum MRE and 23 May 2025 with regard to Colosseum Project Economics. Similarly, the Company confirms that all material assumptions and technical parameters underpinning the estimates and the forecast financial information referred to in those previous announcements continue to apply and have not materially changed.
SOURCE: Dateline Resources Limited
View the original press release on ACCESS Newswire









