Fractyl Health Announces Potent Preclinical Results from RJVA-002, a Dual GIP/GLP-1 Gene Therapy Candidate for Obesity
Rhea-AI Summary
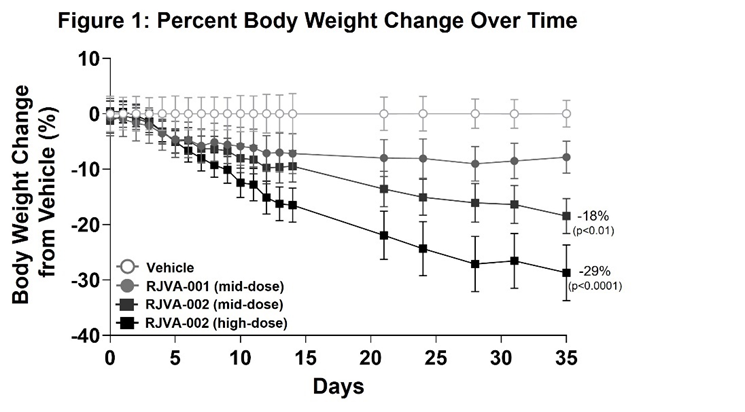
Fractyl Health (Nasdaq: GUTS) reported preclinical data showing a single administration of RJVA-002 produced substantial, dose-dependent weight loss in a diet-induced obesity mouse model with a humanized GIP receptor.
Key measured effects: ~30% weight loss by five weeks (high dose) with trajectory not yet plateaued; mid- and high-dose cohorts lost mean 18% (p<0.01) and 29% (p<0.0001) body weight by day 35; no adverse effects observed. RJVA-002 encodes GIP and GLP-1 under an engineered insulin promoter. RJVA-001 is expected to enter first-in-human trials in 2026.
Positive
- Single-dose RJVA-002 produced ~30% weight loss at five weeks
- Dose-dependent weight loss: mid 18% by day 35, high 29% by day 35
- No adverse effects observed in treated animals
- RJVA-002 targets dual GIP/GLP-1 expression via insulin promoter
Negative
- Results are preclinical and not yet demonstrated in humans
- Small group sizes used: n=7 per cohort
- Longer-term durability and metabolic endpoints remain pending
News Market Reaction – GUTS
On the day this news was published, GUTS declined 6.80%, reflecting a notable negative market reaction. Argus tracked a peak move of +2.6% during that session. Argus tracked a trough of -15.4% from its starting point during tracking. Our momentum scanner triggered 10 alerts that day, indicating notable trading interest and price volatility. This price movement removed approximately $14M from the company's valuation, bringing the market cap to $194M at that time.
Data tracked by StockTitan Argus on the day of publication.
RJVA-002 achieved ~
Preclinical results show that a single point-in-time treatment with RJVA-002 has the potential to achieve significant weight loss that can match or exceed best-in-class chronic drug therapy
BURLINGTON, Mass., Oct. 07, 2025 (GLOBE NEWSWIRE) -- Fractyl Health, Inc. (Nasdaq: GUTS) (the Company), a metabolic therapeutics company focused on pattern-breaking approaches to treat the root causes of obesity and type 2 diabetes (T2D), today announced potent new preclinical data from RJVA-002, the second candidate from the Company’s Rejuva® Smart GLP-1™ platform, at the 2025 Cell & Gene Meeting on the Mesa. The new data expand the potential of the Rejuva platform from the durable treatment of T2D to obesity.
RJVA-002 encodes both GIP and GLP-1 hormones, driven by an engineered human insulin promoter to enable beta cell-specific, nutrient-responsive expression. In a diet-induced obesity (DIO) mouse model with a humanized GIP receptor, a single administration of RJVA-002 led to ~
“These RJVA-002 data further support our Smart GLP-1 gene therapy platform approach and suggest that dual gut hormone expression has the potential to produce powerful metabolic effects,” said Harith Rajagopalan, M.D., Ph.D., Co-Founder and Chief Executive Officer of Fractyl Health. “Together with the recently reported REMAIN-1 data, and with RJVA-001 nearing the clinic in 2026, these results underscore the strength and breadth of our portfolio. We are advancing a multi-modality strategy with the goal of transforming the treatment paradigm for obesity and T2D from chronic disease management toward the durable remission of disease.”
In this ongoing preclinical study, male mice engineered to express a humanized GIP receptor (Biocytogen) were fed a
Results from this ongoing study at longer time points and with associated metabolic measurements will be presented at an upcoming scientific congress.
Rejuva is Fractyl’s gene therapy platform designed to enable long-term remission of T2D and obesity by durably reprogramming pancreatic islet cells to endogenously produce metabolic hormones. RJVA-001, the first Rejuva candidate, is expected to enter first-in-human clinical trials in 2026 for patients with inadequately controlled T2D. RJVA-002 expands the platform into obesity, targeting dual incretin biology with the goal of achieving durable, well-tolerated, weight loss from a single intervention.
The presentation is available via the Investor Relations section of the Fractyl website.
About Fractyl Health
Fractyl Health is a metabolic therapeutics company focused on pioneering new approaches to the treatment of metabolic diseases, including obesity and T2D. Despite advances in treatment over the last 50 years, obesity and T2D continue to be rapidly growing drivers of morbidity and mortality in the 21st century. Fractyl’s goal is to transform metabolic disease treatment from chronic symptomatic management to durable disease-modifying therapies that target the organ-level root causes of disease. The Company has a robust and growing IP portfolio, with 33 granted U.S. patents and approximately 40 pending U.S. applications, along with numerous foreign issued patents and pending applications. Fractyl is based in Burlington, MA. For more information, visit www.fractyl.com.
About Rejuva®
Fractyl Health’s Rejuva platform focuses on developing next-generation adeno-associated virus (AAV)-based, locally delivered gene therapies for the treatment of obesity and T2D. The Rejuva platform is in preclinical development and has not yet been evaluated by regulatory agencies for investigational or commercial use. Rejuva leverages advanced delivery systems and proprietary screening methods to identify and develop metabolically active gene therapy candidates targeting the pancreas. The program aims to transform the management of metabolic diseases by offering novel, disease-modifying therapies that address the underlying root causes of disease. The Company has submitted the first Clinical Trial Application (CTA) module for RJVA-001 in T2D to regulators, and if the CTA is authorized, the Company expects to dose the first patients with RJVA-001 and report preliminary data in 2026. RJVA-002, the Company’s second candidate from the Rejuva platform, is a dual GIP/GLP-1 gene therapy for obesity that has demonstrated approximately
About Revita®
Fractyl Health’s lead product candidate, Revita, is based on the company’s insights surrounding the potential role of the gut in obesity. Revita is designed to remodel the duodenal lining via hydrothermal ablation (i.e. duodenal mucosal resurfacing) to reverse damage to intestinal nutrient sensing and signaling mechanisms caused by chronic high-fat and high-sugar diets that are a root cause of metabolic disease. In the U.S., Revita is for investigational use only under U.S. law. Revita has U.S. FDA Breakthrough Device designation in weight maintenance for people with obesity who discontinue GLP-1 drugs. A pivotal study of Revita in patients with obesity after discontinuation of GLP-1 drugs, called REMAIN-1, was initiated in the third quarter of 2024 and has completed enrollment.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements regarding the promise and potential impact of our preclinical or clinical trial data, the design, initiation, timing, primary and secondary endpoints, and results of clinical enrollment and any clinical studies or readouts, the content, information used for, timing or results of any investigational new drug (IND)-enabling studies, IND applications or Clinical Trial Applications, communications with regulators, the potential launch or commercialization of any of our product candidates or products, the potential treatment population or benefits for any of our product candidates or products, and our strategic and product development objectives and goals, including with respect to enabling long-term control over obesity and type 2 diabetes without the burden of chronic therapies, redefining the future of metabolic disease treatment, positioning our Company at the forefront of the global opportunity for metabolic care, and the timing of any of the foregoing. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause the Company’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the Company’s limited operating history; the incurrence of significant net losses and the fact that the Company expects to continue to incur significant net losses for the foreseeable future; the Company’s need for substantial additional financing; the Company’s ability to continue as a going concern; the restrictive and financial covenants in the Company’s credit agreement; the lengthy and unpredictable regulatory approval process for the Company’s product candidates; uncertainty regarding its clinical studies; the fact that the Company’s product candidates may cause serious adverse events or undesirable side effects or have other properties that may cause it to suspend or discontinue clinical studies, delay or prevent regulatory development, prevent their regulatory approval, limit the commercial profile, or result in significant negative consequences; additional time may be required to develop and obtain regulatory approval or certification for the Company’s Rejuva gene therapy candidates; the Company’s reliance on third parties to conduct certain aspects of the Company’s preclinical studies and clinical studies; the Company’s reliance on third parties for the manufacture of the materials for its Rejuva gene therapy platform for preclinical studies and its ongoing clinical studies; the regulatory approval process of the FDA, comparable foreign regulatory authorities and lengthy, time-consuming and inherently unpredictable, and even if we complete the necessary clinical studies, we cannot predict when, or if, we will obtain regulatory approval or certification for any of our product candidates, and any such regulatory approval or certification may be for a more narrow indication than we seek; and the potential launch or commercialization of any of Company’s product candidates or products and our strategic and product development objectives and goals, and the other factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (the SEC) on August 12, 2025, and in our other filings with the SEC. These forward-looking statements are based on management’s current estimates and expectations. While the Company may elect to update such forward-looking statements at some point in the future, the Company disclaims any obligation to do so, even if subsequent events cause its views to change.
Contacts
Media Contact
Jessica Cotrone, Corporate Communications
jcotrone@fractyl.com, 978.760.5622
Investor Contact
Brian Luque, Head of Investor Relations and Corporate Development
IR@fractyl.com, 951.206.1200
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/265dea50-4fff-4e1e-98d2-3a9c8b346220








