Hologic Announces First and Only FDA-Cleared Digital Cytology System – Genius™ Digital Diagnostics System
Through a Combination of Advanced Imaging and Novel Artificial Intelligence, Latest Diagnostic System for Cervical Cancer Screening Can Help More Accurately Detect Disease, Improve Workflow and Enhance Patient Care
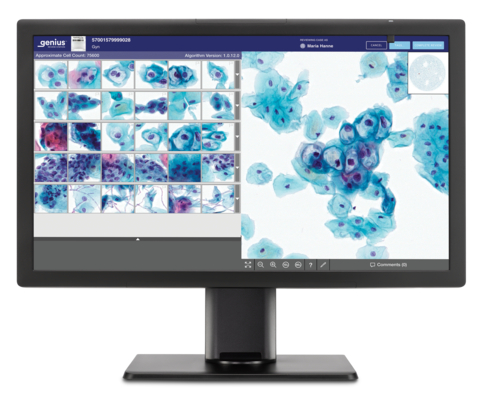
Genius™ Digital Diagnostics System Station (Graphic: Business Wire)
“Hologic is a leading innovator in women’s health with a commitment to advancing cervical and breast cancer screening technologies, from the first liquid-based cytology test to the first 3D mammography system and now the first FDA-cleared digital cytology platform,” said Jennifer Schneiders, Ph.D., President, Diagnostic Solutions at Hologic. “Our technologies have had a tremendous impact on decreasing cancer rates in women, and we are incredibly excited by the promise of Genius Digital Diagnostics. The system delivers more actionable and accurate insights for laboratories and healthcare professionals to enhance patient care.”
In its most recent update, the American Cancer Society estimated that 13,820 cases of invasive cervical cancer will be diagnosed in
Screenings for cervical cancer include a Pap test, where a sample is generally collected at an OB-GYN office, and the cervical cells are sent to a lab where they are transferred to a glass slide. To date, this glass slide has been reviewed under a microscope. With the Genius Digital Diagnostics System, the glass slides are digitally imaged and an artificial intelligence algorithm is applied to pinpoint the cells that cytologists and pathologists should review.
The new process and technology demonstrated an overall improvement in sensitivity without a corresponding decrease in specificity. Notably, there was a
The Genius Digital Diagnostics System also offers the opportunity for greater collaboration across lab and other healthcare settings. The system allows cytologists and pathologists to securely review cases remotely, so patients can benefit from the collective knowledge of geographically dispersed experts.
The Genius Digital Diagnostics System consists of the Genius™ Digital Imager for image acquisition, the Genius™ Cervical AI algorithm for image analysis, the Genius™ Image Management Server for image storage and the Genius™ Review Station for local or remote case review. The complete system is scalable and designed to fit the present and future needs of laboratories. The Genius Digital Diagnostics System is already commercially available in
About Cervical Cancer Screening
Cervical cancer is preventable and, if caught early, can be highly treatable. Co-testing — combining a Pap test with an HPV test — has been shown to be the most sensitive testing option for cervical cancer screening compared to either test used alone.2,3,4,5 Hologic pioneered the first FDA-approved liquid-based cytology test, the ThinPrep® Pap test, and the first FDA-approved mRNA-based HPV test, the Aptima® HPV Assay. Healthcare professionals have the choice to perform a co-test with ThinPrep and Aptima.
About Hologic, Inc.
Hologic, Inc. is a global medical technology innovator focused on improving the health and well-being of women, their families and communities through early detection and treatment. Its advancements include invention of the world’s first commercial 3D mammography system to find breast cancer earlier; leadership in testing for cervical cancer, sexually transmitted infections and respiratory illnesses; minimally invasive surgical technologies for uterine fibroids and abnormal uterine bleeding; and advanced vessel sealing and dissection devices.
The company also champions women through the Hologic Global Women’s Health Index, which provides a science-backed data framework for improving women’s well-being.
Forward-Looking Statements
This press release may contain forward-looking information that involves risks and uncertainties, including statements about the use of Hologic products. There can be no assurance these products will achieve the benefits described herein or that such benefits will be replicated in any particular manner with respect to an individual patient, as the actual effect of the use of the products can only be determined on a case-by-case basis. In addition, there can be no assurance that these products will be commercially successful or achieve any expected level of sales. Hologic expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any such statements presented herein to reflect any change in expectations or any change in events, conditions or circumstances on which any such statements are based.
Hologic, The Science of Sure, Aptima, Genius and ThinPrep and associated logos are trademarks and/or registered trademarks of Hologic, Inc. and/or its subsidiaries in
Source: Hologic, Inc.
| ____________________ | ||
1 |
Genius Digital Diagnostics System with the Genius Cervical AI Algorithm Instructions for Use AW-23890-001. Hologic, Inc.; 2024 |
|
2 |
Austin RM, et al. Enhanced detection of cervical cancer and precancer through use of imaged liquid-based cytology in routine cytology and HPV co-testing. Am J Clin Pathol.2018;150(5):385-392. doi: 10.1093/ajcp/aqy114 (Study included the ThinPrep® Pap test, ThinPrep imaging, digene HPV, Cervista HPV and Aptima HPV). |
|
3 |
Blatt AJ, et al. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123(5):282-288. doi:10.1002/ cncy.21544 (Study included ThinPrep, SurePath and Hybrid Capture 2 assay). |
|
4 |
Kaufman H, et al. Contributions of Liquid-Based (Papanicolaou) Cytology and Human Papillomavirus Testing in Cotesting for Detection of Cervical Cancer and Precancer in |
|
5 |
Zhou H, et al. Clinical performance of the Food and Drug Administration-Approved high-risk HPV test for the detection of high-grade cervicovaginal lesions. Cancer Cytopathol. 2016 May;124(5):317-23. doi: 10.1002/cncy.21687. (Study included Cobas HPV, SurePath and ThinPrep). |
|
View source version on businesswire.com: https://www.businesswire.com/news/home/20240131786693/en/
Media Contact:
Bridget Perry
Director, Corporate Communications
+1 508.263.8654
bridget.perry@hologic.com
Investor Contact:
Ryan M. Simon
Vice President, Investor Relations
+1 858.410.8514
ryan.simon@hologic.com
Source: Hologic, Inc.









