Immuron Reports Continued Sales Growth
Rhea-AI Summary
Immuron (NASDAQ: IMRN) reported continued unaudited sales growth for Travelan® in H1 FY26 with global H1 sales of AUD$4.2 million, up 5% on the prior comparative period. Key regional results included Australia H1 AUD$3.3 million, +13%; USA H1 AUD$0.9 million, +17%; and Canada H1 AUD$56 thousand, -85% while Canada Q2 recovered +191% quarter-on-quarter. Growth in Australia and the U.S. was attributed to expanded digital/social marketing, promotions, new store listings and increased travel; a one-off reduction in stock holdings from the merged Sigma Healthcare/Chemist Warehouse group offset some Australian gains. ProIBS® launched in Australia before Christmas and is listed with two of three major pharmacy wholesalers and five banner groups.
Positive
- Global H1 sales AUD$4.2M, +5% on pcp
- Australia H1 sales AUD$3.3M, +13% on pcp
- USA H1 sales AUD$0.9M, +17% on pcp
- Canada Q2 sales +191% on prior quarter
- ProIBS® launched early and listed in five banner groups
Negative
- Canada H1 sales AUD$56k, down 85% on pcp
- One-off stock reduction from merged Sigma Healthcare/Chemist Warehouse group offset Australian growth
- Launch timing close to year end limited initial ProIBS® rollout opportunities
News Market Reaction – IMRN
On the day this news was published, IMRN declined 7.83%, reflecting a notable negative market reaction. Our momentum scanner triggered 2 alerts that day, indicating moderate trading interest and price volatility. This price movement removed approximately $735K from the company's valuation, bringing the market cap to $9M at that time.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
IMRN was down 0.93% while highlighted biotech peers showed mixed moves, from -17.57% (ADAP) to +3.93% (XTLB), pointing to stock-specific dynamics rather than a broad sector trend.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Dec 03 | Defense award & trial | Positive | -10.9% | DoD-funded program and Travelan clinical timeline update. |
| Nov 05 | FDA IND approval | Positive | -12.7% | FDA IND clearance enabling Phase 2 IMM-529 trial. |
| Oct 31 | Clinical update | Positive | -5.9% | IMM-529 review status and Travelan P2TD timing change. |
| Oct 13 | Sales update | Positive | +0.5% | Q1 FY26 sales growth across Australia and U.S. |
| Oct 08 | IND submission | Positive | +2.7% | Submission of IND to FDA for IMM-529 Phase 2 trial. |
Recent positive clinical and growth updates often coincided with negative price reactions, indicating a pattern of selling into good news.
Over the last few months, Immuron reported multiple clinical and commercial milestones. On Oct 8, 2025, it submitted an IND for IMM-529 with Phase 2 plans and cited potential US$400M annual revenue, followed by an FDA IND approval on Nov 5, 2025. Travelan clinical updates and a U.S. Department of Defense-funded program were announced on Oct 31 and Dec 3, 2025. Commercially, Q1 FY26 unaudited sales reached AUD$2.0M with strong Australia and U.S. growth. Today’s H1 sales growth release extends that commercial momentum theme.
Market Pulse Summary
The stock moved -7.8% in the session following this news. A negative reaction despite H1 sales growth of AUD$4.2 million and solid Australia/U.S. trends would fit a recent pattern where positive announcements sometimes preceded declines. Investors could be weighing past dilution and the weak Canadian H1 result of AUD$56 thousand (down 85% on pcp). Future price stability may hinge on consistent revenue execution and clarity around funding needs.
Key Terms
gastrointestinal (gi) tract medical
hyper-immune bovine antibodies medical
travelers’ diarrhea medical
australian register for therapeutic goods regulatory
natural health product regulatory
AI-generated analysis. Not financial advice.
Sales Highlights (unaudited):
| Global |
|
| Australia |
|
| Canada |
|
| USA |
|
MELBOURNE, Australia, Jan. 19, 2026 (GLOBE NEWSWIRE) -- Immuron Limited (ASX: IMC; NASDAQ: IMRN), an Australian based and globally integrated biopharmaceutical company is pleased to announce continued sales growth (unaudited) of Travelan®, an over-the-counter immune supplement that targets pathogenic bacteria and the toxins they produce in the gastrointestinal (GI) tract.
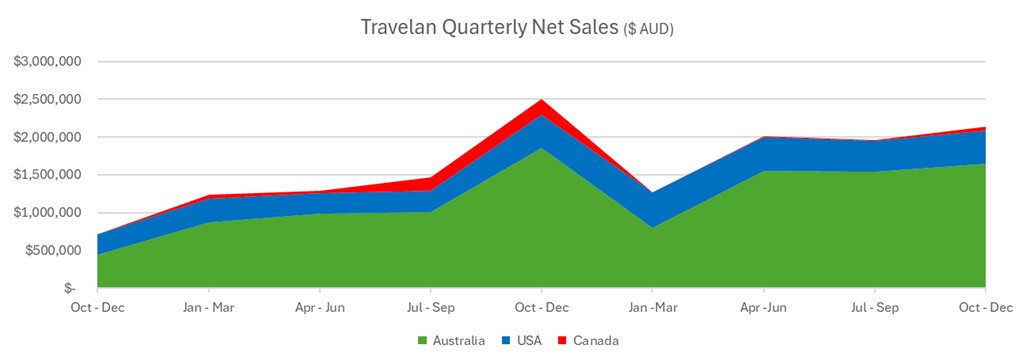
Continued Travelan® H1 sales growth (+
Although it is difficult launching close to year end, we managed to launch ProIBS® (https://investors.immuron.com.au/announcements/7225649) prior to Christmas ahead of schedule to gain some early opportunities. ProIBS® is stocked by two of the three largest pharmacy wholesalers with listings in five banner groups. 2026 category reviews provide us with the opportunity for additional listings. We believe that once Australian consumers try ProIBS®, they will love it!
H1 sales in the U.S. increased (+
During FY25 we had a Q1 pipeline fill into over a thousand Canada retail doors on the back of securing listings within key pharmacy and grocery retail groups. As expected, sales picked up on the back of consumer promotions in Q2 FY26 (+
Travelan® will be launched into Jean Coutu pharmacies in 3QFY26. Jean Coutu is the number one or two pharmacy group in Quebec, the second largest province in Canada by population.
This release has been authorised by the directors of Immuron Limited.
| COMPANY CONTACT: Steven Lydeamore Chief Executive Officer steve@immuron.com |
About Travelan®
Travelan® is an orally administered passive immunotherapy that prophylactically reduces the likelihood of contracting travelers’ diarrhea, a digestive tract disorder that is commonly caused by pathogenic bacteria and the toxins they produce. Travelan® is a purified tablet preparation of hyper-immune bovine antibodies and other factors, which when taken with meals bind to diarrhea-causing bacteria and prevent colonization and the pathology associated with traveler’s diarrhea. In Australia, Travelan® is a listed medicine on the Australian Register for Therapeutic Goods (AUST L 106709) and is indicated to reduce the risk of Traveler’s Diarrhea, reduce the risk of minor gastro-intestinal disorders and is antimicrobial. In Canada, Travelan® is a licensed natural health product (NPN 80046016) and is indicated to reduce the risk of Traveler’s Diarrhea. In the U.S., Travelan® is sold as a dietary supplement for digestive tract protection.
About Immuron
Immuron Limited (ASX: IMC, NASDAQ: IMRN), is an Australian biopharmaceutical company focused on developing and commercializing orally delivered targeted polyclonal antibodies for the treatment of inflammatory mediated and infectious diseases.
Immuron Platform Technology
Immuron’s proprietary technology is based on polyclonal immunoglobulins (IgG) derived from engineered hyper-immune bovine colostrum. Immuron has the capability of producing highly specific immunoglobulins to any enteric pathogen and our products are orally active. Bovine IgG can withstand the acidic environment of the stomach and is resistant to proteolysis by the digestive enzymes found in the Gastrointestinal (GI) tract. Bovine IgG also possesses this unique ability to remain active in the human GI tract delivering its full benefits directly to the bacteria found there. The underlying nature of Immuron’s platform technology enables the development of medicines across a large range of infectious diseases. The platform can be used to block viruses or bacteria at mucosal surfaces such as the Gastrointestinal tract and neutralize the toxins they produce.
For more information visit: https://www.immuron.com.au/ and https://www.travelan.com
Sign up to Immuron’s Investor Hub: Here
FORWARD-LOOKING STATEMENTS:
This press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, each as amended. Such statements include, but are not limited to, any statements relating to our growth strategy and product development programs and any other statements that are not historical facts. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock value. Factors that could cause actual results to differ materially from those currently anticipated include: risks relating to our growth strategy; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; risks relating to the results of research and development activities; risks relating to the timing of starting and completing clinical trials; uncertainties relating to preclinical and clinical testing; our dependence on third-party suppliers; our ability to attract, integrate and retain key personnel; the early stage of products under development; our need for substantial additional funds; government regulation; patent and intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/576036f2-3c0a-4722-bbfc-02dd4072e96e








