Innovent Announces Phase 2 Results of Tigulixostat (IBI128, XOI) in Gout Patients were Published at the 27th Asia-Pacific League of Associations for Rheumatology Congress
Rhea-AI Summary
Innovent Biologics (IVBIY) has announced positive Phase 2 clinical trial results for tigulixostat (IBI128), its novel xanthine oxidase inhibitor for gout treatment, at the 2025 APLAR Congress. The study involved 84 Chinese participants comparing three tigulixostat doses (50mg, 100mg, 200mg) against febuxostat 40mg over 16 weeks.
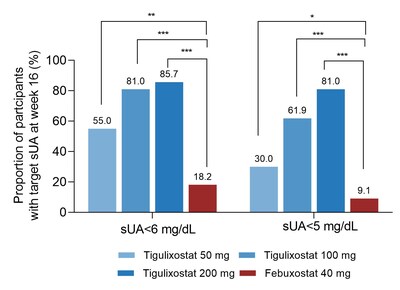
The results showed significantly superior urate-lowering efficacy across all tigulixostat dose groups compared to febuxostat. At week 16, the proportion of participants achieving target serum uric acid levels ranged from 55.0% to 85.7% for tigulixostat groups, versus 18.2% for febuxostat. The drug demonstrated a favorable safety profile with no serious adverse events. Based on these positive results, Innovent plans to initiate Phase 3 trials in China in H2 2025.
Positive
- Superior efficacy with 85.7% of patients in 200mg group achieving target uric acid levels vs 18.2% for febuxostat
- Demonstrated favorable safety profile with no serious adverse events
- Dose-dependent effectiveness across all treatment groups
- No increased risk of renal impairment observed
- Strong reduction in serum uric acid levels up to -57.11% from baseline
Negative
- Gout flares occurred during initial 4 weeks of treatment, though comparable to control group
- Study was open-label rather than double-blind, which may introduce bias
News Market Reaction – IVBIY
On the day this news was published, IVBIY gained 10.45%, reflecting a significant positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
The published results are based on a randomized, open-label, multicenter, parallel-group, active-controlled Phase 2 clinical study (NCT06501534) evaluating the efficacy and safety of different doses of tigulixostat in Chinese gout participants.
A total of 84 participants (mean age: 37 years; mean baseline serum uric acid [sUA]: 575 μmol/L [9.6 mg/dL]; mean baseline weight: 80.7 kg) were enrolled and randomized to receive tigulixostat 50 mg, 100 mg, 200 mg or febuxostat 40 mg for 16 weeks (or 18 weeks for the tigulixostat 200 mg group). The primary endpoint was the proportion of participants achieving sUA <360 μmol/L (6 mg/dL) at Week 16.
Tigulixostat demonstrated significantly greater urate-lowering efficacy compared to febuxostat across all dose groups, with a dose-dependent effect.
- At week 16,
55.0% of participants in the tigulixostat 50 mg group,81.0% in the tigulixostat 100 mg group, and85.7% in the tigulixostat 200 mg group, achieved sUA <360 μmol/L (6 mg/dL), respectively, compared with18.2% in the febuxostat 40 mg group, the treatment difference versus febuxostat was36.8% (95% CI: 9.7, 63.9, P<0.01),62.8% (39.5, 86.0, P<0.001), and67.5% (45.5, 89.5, P<0.001) for the tigulixostat 50 mg group, 100 mg group, and 200 mg group, respectively; - At week 16,
30.0% of participants in the tigulixostat 50 mg group,61.9% in the tigulixostat 100 mg group, and81.0% in the tigulixostat 200 mg group, achieved sUA <300 μmol/L (5 mg/dL), respectively, compared with9.1% in the febuxostat 40 mg group, the treatment difference versus febuxostat was20.9% (95% CI: –2.5, 44.3, P<0.05),52.8% (28.8, 76.8, P<0.001), and71.9% (51.2, 92.5, P<0.001) for the tigulixostat 50 mg group, 100 mg group, and 200 mg group, respectively. - At week 16, the percent change in sUA from baseline were -
38.66% in the tigulixostat 50 mg group, -48.61% in the tigulixostat 100 mg group、-57.11% in the tigulixostat 200 mg group, and -24.11% (in the febuxostat 40 mg group. - Tigulixostat demonstrated a favorable safety profile, no increased risk of renal impairment. The incidence of adverse events was similar across groups. All reported AEs were mild to moderate in severity. No serious adverse events or adverse events of special interest were observed in any tigulixostat -treated groups. No adverse events led to temporary or permanent discontinuation of the study drug was observed.
- Without any gout flare prophylaxis during the study, the incidences of gout flare were comparable between the tigulixostat total group and the febuxostat 40 mg group. Most gout flare occurred during the initial 4 weeks of treatment, with the incidence gradually decreasing and reaching a plateau subsequently. It has been reported that higher and more sustained serum uric acid target achievement can significantly reduce the risk of gout flare[1].
The Principal Investigator of the Study, Professor Hejian Zou from Huashan Hospital affiliated with Fudan University, stated: "Gout and hyperuricemia are increasingly becoming the 'fourth major' chronic disease following hypertension, hyperlipidemia, and hyperglycemia. The overall prevalence of hyperuricemia in
Dr. Lei Qian, Chief R&D Officer of General Biomedicine from Innovent Biologics, stated, "We are delighted to present the Phase 2 clinical study results of tigulixostat at the international academic congress. The study demonstrated tigulixostat's superior urate-lowering efficacy compared to febuxostat, while maintaining a favorable safety profile across multiple dose levels in Chinese gout participants. Building on these findings and through ongoing communication with regulatory authorities, we will accelerate the Phase 3 clinical development of tigulixostat to bring this promising treatment option to Chinese gout patients as soon as possible. As part of our commitment, Innovent Biologics will continue to advance our next-generation pipeline in cardiovascular and metabolic diseases, targeting the broad population affected by hypertension, hyperlipidemia, hyperglycemia, and hyperuricemia. We remain dedicated to fulfilling patients' aspirations for healthier lives and expanding access to innovative therapies."
About Gout
Gout is an inflammatory arthritis caused by the gradual elevation of uric acid levels in the blood, exceeding its solubility threshold[2], leading to the deposition of monosodium urate (MSU) crystals in joints. Persistent hyperuricemia can result in the accumulation of urate crystals (tophi) in joints, tendons, and other tissues, which, under triggering factors (e.g., trauma, cold exposure, inflammatory stimuli), may provoke acute inflammatory episodes known as acute gout attacks.
Hyperuricemia is a metabolic disorder syndrome resulting from purine metabolism dysfunction. It is defined as serum uric acid levels exceeding 420 μmol/L on two separate occasions, regardless of gender. Hyperuricemia and gout represent a continuous, chronic pathophysiological spectrum and constitute a systemic disease affecting multiple organ systems. The prevalence of hyperuricemia varies across ethnicities, ranging from
The most widely prescribed urate-lowering drugs in
About Tigulixostat (IBI128)
Tigulixostat (IBI128) is a next-generation, non-purine selective xanthine oxidase inhibitor (XOI). It exerts its therapeutic effect by inhibiting xanthine oxidase activity, thereby blocking the conversion of hypoxanthine and xanthine to uric acid. This mechanism reduces uric acid production and subsequently lowers serum uric acid levels, making it an effective treatment for hyperuricemia in gout patients.
Currently, tigulixostat has completed a Phase 2 clinical study in
In December 2022, Innovent Biologics entered a strategic collaboration with LG Chem, securing exclusive development and commercialization rights for tigulixostat in
About Innovent
Innovent is a leading biopharmaceutical company founded in 2011 with the mission to empower patients worldwide with affordable, high-quality biopharmaceuticals. The company discovers, develops, manufactures and commercializes innovative medicines that target some of the most intractable diseases. Its pioneering therapies treat cancer, cardiovascular and metabolic, autoimmune and eye diseases. Innovent has launched 16 products in the market. It has 2 new drug applications under regulatory review, 4 assets in Phase 3 or pivotal clinical trials and 15 more molecules in early clinical stage. Innovent partners with over 30 global healthcare companies, including Eli Lilly, Sanofi, Incyte, LG Chem and MD Anderson Cancer Center.
Guided by the motto, "Start with Integrity, Succeed through Action," Innovent maintains the highest standard of industry practices and works collaboratively to advance the biopharmaceutical industry so that first-rate pharmaceutical drugs can become widely accessible. For more information, visit www.innoventbio.com, or follow Innovent on Facebook and LinkedIn.
Statement: Innovent does not recommend the use of any unapproved drug (s)/indication (s).
Forward-looking statement
This news release may contain certain forward-looking statements that are, by their nature, subject to significant risks and uncertainties. The words "anticipate", "believe", "estimate", "expect", "intend" and similar expressions, as they relate to Innovent Biologics ("Innovent"), are intended to identify certain of such forward-looking statements. The Company does not intend to update these forward-looking statements regularly.
These forward-looking statements are based on the existing beliefs, assumptions, expectations, estimates, projections and understandings of the management of the Company with respect to future events at the time these statements are made. These statements are not a guarantee of future developments and are subject to risks, uncertainties and other factors, some of which are beyond the Company's control and are difficult to predict. Consequently, actual results may differ materially from information contained in the forward-looking statements as a result of future changes or developments in our business, the Company's competitive environment and political, economic, legal and social conditions.
The Company, the Directors and the employees of the Company assume (a) no obligation to correct or update the forward-looking statements contained in this site; and (b) no liability in the event that any of the forward-looking statements does not materialise or turn out to be incorrect.
Reference: |
[1] Lisa K Stamp et al., (2021). Association between serum urate and flares in peoplewith gout and evidence for surrogate status: a secondary analysis of tworandomised controlled trials. The Lancet Rheumatology. DOI: https://doi.org/10.1016/S2665-9913(21)00319-2 |
Martillo MA, Nazzal L, Crittenden DB. (2014). The crystallization of monosodium urate. Current Rheumatol Rep.16(2):400. |
[2] Chinese Society of Endocrinology, Chinese Medical Association. 2019 Chinese Guidelines for the Diagnosis and Management of Hyperuricemia and Gout. Chinese Journal of Endocrinology and Metabolism, 2020, 36(01): 1-13. |
[3] 中华医学会内分泌学分会. 2019年中国高尿酸血症与痛风诊疗指南. 中华内分泌代谢杂志, 2020,36(01) : 1-13. |
[4] Hyndman D, Liu S, Miner JN. (2016). Urate handling in the human body. Curr Rheumatol Rep. 18(6):34. |
[5] Sattui SE, Gaffo AL. (2016). Treatment of hyperuricemia in gout: current therapeutic options, latest developments and clinical implications. Ther Adv Musculoskelet Dis. 8(4):145-159. |
[6] White WB, Saag KG, Becker MA, et al. (2018). Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 378:1200-1210. |
[7] Prescribing Information ULORIC (febuxostat) tablet for oral use https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021856s013lbl.pdf Accessed 2 Jun 2019. |