NurExone’s Exosomes Show Stronger Healing Potential Than Industry Standard
Rhea-AI Summary
NurExone Biologic (OTCQB: NRXBF) announced that an independent study by TAmiRNA laboratory revealed their exosomes significantly outperformed commercial industry standards in key healing capabilities. The company's exosomes demonstrated approximately 1.8x higher neurological potential, 2x higher tissue-regeneration and wound-healing signals, and nearly double the anti-inflammatory activity compared to benchmark products.
The analysis, conducted on exosomes produced from NurExone's proprietary master cell bank, validates the company's core technology for therapeutic nerve repair and aesthetic applications. The exosomes will be manufactured by NurExone's U.S. subsidiary, ExoTOP Inc., using their patent-pending 3D production process.
Positive
- Independent study confirms superior performance versus industry benchmark across all healing pathways
- Exosomes show 1.8x higher neurological potential and 2x higher tissue-regeneration capability
- Patent-pending 3D production process ensures reproducible high-performance exosomes
- Technology validated for both therapeutic and aesthetic applications
Negative
- Production scaling and commercialization still pending
- Revenue generation from ExoTOP subsidiary yet to materialize
News Market Reaction
On the day this news was published, NRXBF gained 4.42%, reflecting a moderate positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
Independent Study and Bioanalysis Suggests More Effective Nerve Repair and Faster Wound Healing
TORONTO and HAIFA, Israel, Aug. 08, 2025 (GLOBE NEWSWIRE) -- NurExone Biologic Inc. (TSXV: NRX) (OTCQB: NRXBF) (FSE: J90) (“NurExone” or the “Company”) today announced that an independent study showed that exosomes produced by NurExone outperformed a recognized commercial industry standard in areas that strongly support key healing tasks including nerve repair, wound repair, calming the immune system and rebuilding tissue. This first independent analysis highlighted the broad potential of NurExone’s exosomes for both therapeutic and aesthetics markets.
“This data confirms that the naïve exosomes that will be manufactured by our U.S. subsidiary, ExoTOP Inc. (“ExoTOP”), will carry a strong regenerative and therapeutic punch. Delivering more than twice the wound-healing signals than the industry benchmark suggests applications in aesthetic skin rejuvenation, wound care and orthopedic tissue repair”, said Jacob Licht, Chief Executive Officer of ExoTOP. “As we scale production in the United States with our patent-pending 3D production process, ExoTOP is expected to generate multiple revenue streams and provide high-performance exosomes to partners across regenerative medicine.”
“Our exosomes carry complex cargo with diverse therapeutic potential, simultaneously being effective in neuroprotection and reduction in inflammation,” said Dr. Tali Kizhner, Director of R&D of NurExone Biologic. “This combination is especially powerful in the nervous system, where inflammation usually prevents healing. The benchmarking analysis confirms that our exosomes naturally carry a significant amount of the molecular signals needed to create the conditions required for meaningful nerve regeneration.”
TAmiRNA, an ISO 13485‑certified molecular‑diagnostics laboratory, performed a comprehensive analysis of the EV microRNA cargo of NurExone’s exosomes and compared the results with benchmark exosomes from a commercial reference source. Based on TAmiRNA’s data, bioinformatic analyses showed that NurExone’s exosomes are enriched with microRNAs that support key healing tasks.
Importantly, these exosomes were produced from NurExone’s proprietary master cell bank, whose cells are maintained under rigorously controlled environment ensuring that every production batch delivers high-performance exosomes. This reproducibility is essential for clinical translation and future patient use.
Figure 1 suggests that NurExone’s naïve exosomes outperform the commercial reference across every pathway assessed: they exhibit ≈1.8-fold higher neurological potential, nearly double the anti-inflammatory activity, and a full two-fold increase in both tissue-regeneration and wound-healing signals. This multi-modal, high-potency profile positions the exosomes as a versatile platform for therapeutic nerve repair as well as for aesthetic regenerative and longevity applications.
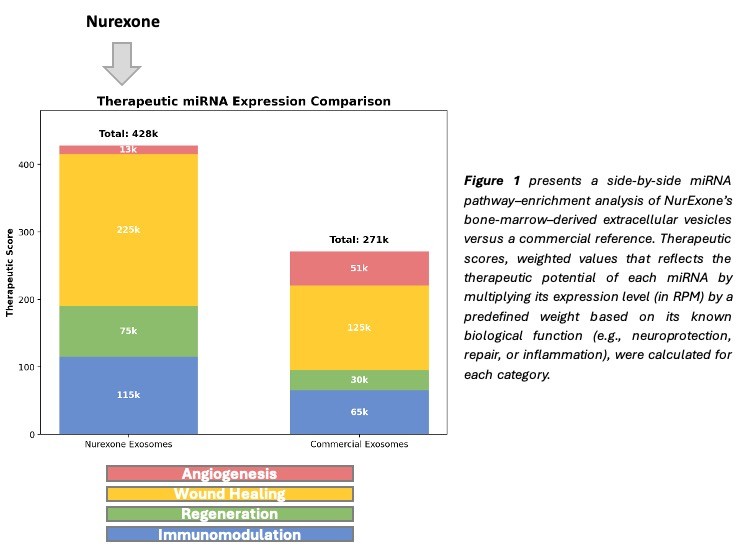
The results provide strong, third-party validation of NurExone’s core technology and highlight its potential across a broad range of regenerative-medicine applications.
About TAmiRNA GmbH
Vienna‑based TAmiRNA is an ISO 13485‑certified molecular‑diagnostics laboratory whose CE‑marked biomarker kits and miND® small‑RNA sequencing platform provide industry‑leading, ISEV‑compliant exosome analytics. Its extensive extracellular‑vesicle reference database and bench‑to‑algorithm workflow deliver regulatory‑grade, reproducible insights for precision‑medicine programs.
About NurExone
NurExone Biologic Inc. is a TSX Venture Exchange (“TSXV”), OTCQB, and Frankfurt-listed biotech company focused on developing regenerative exosome-based therapies for central nervous system injuries. Its lead product, ExoPTEN, has demonstrated strong preclinical data supporting clinical potential in treating acute spinal cord and optic nerve injury, both multi-billion-dollar marketsi . Regulatory milestones, including obtaining the Orphan Drug Designation, facilitates the roadmap towards clinical trials in the U.S. and Europe. Commercially, the Company is expected to offer solutions to companies interested in quality exosomes and minimally invasive targeted delivery systems for other indications. NurExone has established Exo-Top Inc., a U.S. subsidiary, to anchor its North American activity and growth strategy.
For additional information and a brief interview, please watch Who is NurExone?, visit www.nurexone.com or follow NurExone on LinkedIn, Twitter, Facebook, or YouTube.
For more information, please contact:
Dr. Lior Shaltiel
Chief Executive Officer and Director
Phone: +972-52-4803034
Email: info@nurexone.com
Dr. Eva Reuter
Investor Relations – Germany
Phone: +49-69-1532-5857
Email: e.reuter@dr-reuter.eu
Allele Capital Partners
Investor Relations – U.S.
Phone: +1 978-857-5075
Email: aeriksen@allelecapital.com
FORWARD-LOOKING STATEMENTS
This press release contains certain “forward-looking statements” that reflect the Company’s current expectations and projections about its future results. Wherever possible, words such as “may”, “will”, “should”, “could”, “expect”, “plan”, “intend”, “anticipate”, “believe”, “estimate”, “predict” or “potential” or the negative or other variations of these words, or similar words or phrases, have been used to identify these forward-looking statements. Forward-looking statements in this press release include, but are not limited to, statements relating to: the broad potential of the Company’s exosomes for both therapeutic and aesthetics markets; naïve exosomes manufactured by ExoTOP carrying a strong regenerative and therapeutic punch; the Company’s expectation that ExoTOP will generate multiple revenue streams and provide high-performance exosomes to partners across regenerative medicine; the reproducibility of the Company’s exosomes being essential for clinical translation and future patient use; third party data highlighting the potential of the Company’s core technology across a range of regenerative-medicine applications; and the NurExone platform technology offering novel solutions to drug companies interested in minimally invasive targeted drug delivery for other indications, including recovery of optic nerve function and overall visual health.
These statements reflect management’s current beliefs and are based on information currently available to management as at the date hereof. In developing the forward-looking statements in this press release, we have applied several material assumptions, including: the Company’s exosomes will have versatile applications supported by evidence displaying their effectiveness in regenerative medicine and aesthetics; the exosomes produced by ExoTOP are capable of delivering therapeutic effects; commercial scalability, market demand, and competitive advantage of ExoTOP’s exosomes; the Company’s exosomes will be therapeutically applied across various clinical trials; the Company’s manufacturing process will consistently produce exosomes to a standard sufficient for regulatory approval and clinical reliability; the Company’s exosomes will be effectively used in regenerative frameworks targeting nerve tissue as well as skin and aesthetics; the Company having credible external validation and supportive data to substantiate the potential of its technology; and the NurExone platform technology has the ability to offer novel solutions to drug companies interested in minimally invasive targeted drug delivery for other indications, including recovery of optic nerve function and overall visual health
Forward-looking statements involve significant risk, uncertainties and assumptions. Many factors could cause actual results, performance or achievements to differ materially from the results discussed or implied in the forward-looking statements. These risks and uncertainties include, but are not limited to risks related to: slow market adoption; unanticipated safety concerns from novel clinical uses; regulatory and manufacturing compliance roadblocks the Company’s early stage of development; lack of revenues to date; the inherent uncertainty of preclinical drug development, including the risk that product candidates may not advance to clinical trials or receive regulatory approval; the possibility that results from preclinical studies and early-stage trials may not predict later outcomes; the uncertain timing, cost, and outcome of preclinical and clinical development activities; risks related to the clinical trial process, including potential delays or failure to achieve effective trial design or positive results; the inability to obtain or maintain required regulatory approvals; limited market acceptance of the Company’s products, even if approved; the potential emergence of competing therapies that are safer, more effective, or more affordable; rapid technological change that may impact the relevance of the Company’s technologies; the Company’s dependence on key personnel and strategic partners; the inability to obtain adequate financing; risks related to the Company’s ability to protect its intellectual property; the possibility that the Company’s technologies, including its exosome-based platforms, may not achieve their intended therapeutic impact; the inability to produce or scale exosome-based products for clinical use; limited adoption in regenerative medicine or cell therapy applications; lack of growing clinical demand in targeted indications such as spinal cord injury, optic nerve repair, or other therapeutic areas; failure to meet planned development milestones or achieve commercial breakthroughs; the Company will not initiate additional studies to explore alternative dosing regimens; the Company will not advance the optimization of ExoPTEN’s manufacturing processes and analytical methods; the NurExone platform technology not offering novel solutions to drug companies interested in minimally invasive targeted drug delivery for other indications, including recovery of optic nerve function and overall visual health; and the risks discussed under the heading “Risk Factors” on pages 44 to 51 of the Company’s Annual Information Form dated August 27, 2024, a copy of which is available under the Company’s SEDAR+ profile at www.sedarplus.ca. These factors should be considered carefully, and readers should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in this press release are based upon what management believes to be reasonable assumptions, the Company cannot assure readers that actual results will be consistent with these forward-looking statements. These forward-looking statements are made as of the date of this press release, and the Company assumes no obligation to update or revise them to reflect new events or circumstances, except as required by law.
Neither the TSXV nor its Regulation Services Provider (as that term is defined in the policies of the TSXV) accepts responsibility for the adequacy or accuracy of this release.
i Spinal cord injury, Glaucoma
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/adb4c1ac-d77e-4955-9ddf-39f41f799d4b








