SAB BIO Announces Positive Confirmatory Clinical Results from the Phase 1 Study of SAB-142 in Development for the Treatment of Stage 3 T1D
Rhea-AI Summary
SAB BIO (Nasdaq: SABS) announced positive confirmatory Phase 1 results for SAB-142, an hATG candidate for stage 3 type 1 diabetes (T1D). In 68 treated participants (healthy volunteers and T1D patients), SAB-142 showed 0% serum sickness (0/68), 0% anti-drug antibody events (0/68), no drug-related serious adverse events, and transient lymphopenia that resolved to baseline within 1–3 days in 100% of subjects (68/68). Most adverse events were mild and infusion-related. A registrational Phase 2b SAFEGUARD trial is underway and recruiting globally, with the company on track to dose the first patient by year-end.
Positive
- 0% serum sickness observed (0/68)
- 0% ADA-related adverse events reported (0/68)
- Transient lymphopenia corrected to baseline within 1–3 days (100%; 68/68)
- No drug-related SAEs in Phase 1 cohorts
- Phase 2b SAFEGUARD trial underway and recruiting globally
Negative
- Only 6 T1D patients included in Phase 1 T1D cohort (n=6)
- No efficacy endpoints or clinical benefit data reported in Phase 1
News Market Reaction
On the day this news was published, SABS declined 0.75%, reflecting a mild negative market reaction. This price movement removed approximately $1M from the company's valuation, bringing the market cap to $188M at that time. Trading volume was above average at 1.7x the daily average, suggesting increased trading activity.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
Peers showed mixed moves, with QTTB down 4.64% while LIXT, NNVC, and NRXS were modestly positive; no broad biotech trend clearly aligned with SABS’ slight -0.25% move.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Dec 08 | Clinical data preview | Positive | -10.8% | Conference presentation of Phase 1 SAB-142 data and development progress. |
| Nov 13 | Earnings and pipeline | Positive | -9.1% | Q3 results, strong cash runway, and SAFEGUARD Phase 2b trial initiation. |
| Nov 04 | Conference presentation | Positive | +0.0% | ISPAD presentations on Phase 1 SAB-142 data and mechanism insights. |
| Nov 04 | Investor conferences | Neutral | +0.0% | Planned participation in several healthcare investor conferences. |
| Sep 19 | Clinical data updates | Positive | -11.5% | EASD presentations on Phase 1 SAB-142 and disease-modifying potential. |
Recent positive scientific and financial updates on SAB-142 often coincided with negative or flat price reactions, suggesting a pattern of weak price follow-through on good news.
Over the last six months, SABS repeatedly highlighted progress for SAB-142, including multiple Phase 1 data presentations and initiation of the Phase 2b SAFEGUARD trial. Events on Sep 19, Nov 4, and Dec 8, 2025 emphasized favorable safety, immunomodulation without sustained lymphodepletion, and conference visibility, yet shares often fell afterward. The Nov 13, 2025 earnings update also paired a strong cash position and runway through 2028 with a negative reaction. Today’s confirmatory Phase 1 results and Phase 2b progression fit this steady clinical advancement narrative.
Market Pulse Summary
This announcement delivered confirmatory Phase 1 data for SAB-142, showing 0% serum sickness, no ADA-related adverse events in 68 participants, and transient lymphopenia resolving within 1–3 days. The results support chronic outpatient dosing and underlie progression into the registrational Phase 2b SAFEGUARD trial for new-onset Stage 3 T1D. In context of prior SAB-142 updates, investors may focus on continued safety consistency, timely Phase 2b enrollment, and the quality of future efficacy data.
Key Terms
serum sickness medical
immunogenicity medical
anti-thymocyte immunoglobulin medical
anti-drug antibodies medical
pharmacodynamic medical
lymphopenia medical
adverse events medical
immunomodulatory drugs medical
AI-generated analysis. Not financial advice.
- Phase 1 data confirms SAB-142 does not cause serum sickness and has low/no immunogenicity at any dose and in all cohorts, including redosed healthy volunteers
- Study results support the chronic dosing of SAB-142 in an outpatient setting for the treatment of stage 3 autoimmune type 1 diabetes
- Phase 2b SAFEGUARD trial underway and recruiting at multiple sites around the world
MIAMI, Dec. 17, 2025 (GLOBE NEWSWIRE) -- SAB Biotherapeutics, Inc. (Nasdaq: SABS), a clinical-stage biopharmaceutical company developing human anti-thymocyte immunoglobulin (hATG) for type 1 diabetes (T1D) and other autoimmune diseases, today announced positive, confirmatory data from a Phase 1 trial of SAB-142 in a single- and multiple-ascending dose among healthy volunteers (n=62), including a re-dosed cohort, and T1D patients (n=6). The study met its primary objectives to establish a safety profile and characterize pharmacodynamic activity enabling SAB-142 to advance to Phase 2b clinical development in the SAFety and Efficacy of human anti-thymocyte immunoGlobUlin SAB-142 ARresting progression of type 1 Diabetes (SAFEGUARD) clinical trial, now underway.
In the Phase 1 trial, nine (9) cohorts of healthy volunteers (HVs) and one (1) cohort of T1D patients were dosed with a single 0.03-4.5mg/kg dose or multiple doses of SAB-142.
SAB-142 was well-tolerated in both healthy volunteers and T1D patients. SAB-142 demonstrates a safety profile superior to rabbit anti-thymocyte immunoglobulin (rATG) as the data from the Phase 1 trial confirmed SAB-142 does not cause serum sickness (
In all treated participants, there were no drug-related serious adverse events (SAE). Most AEs were mild and associated with day 1-2 infusions, with only Grade 1 flu-like symptoms and transient infusion-site reactions including pruritus and tenderness. The most common AE was headache, which is consistent with typical AEs for T-cell modifying therapies.
Transient lymphopenia, an on-target marker of target engagement and pharmacodynamic activity, was observed after dosing and rapidly corrected to baseline within 1-3 days in all subjects (
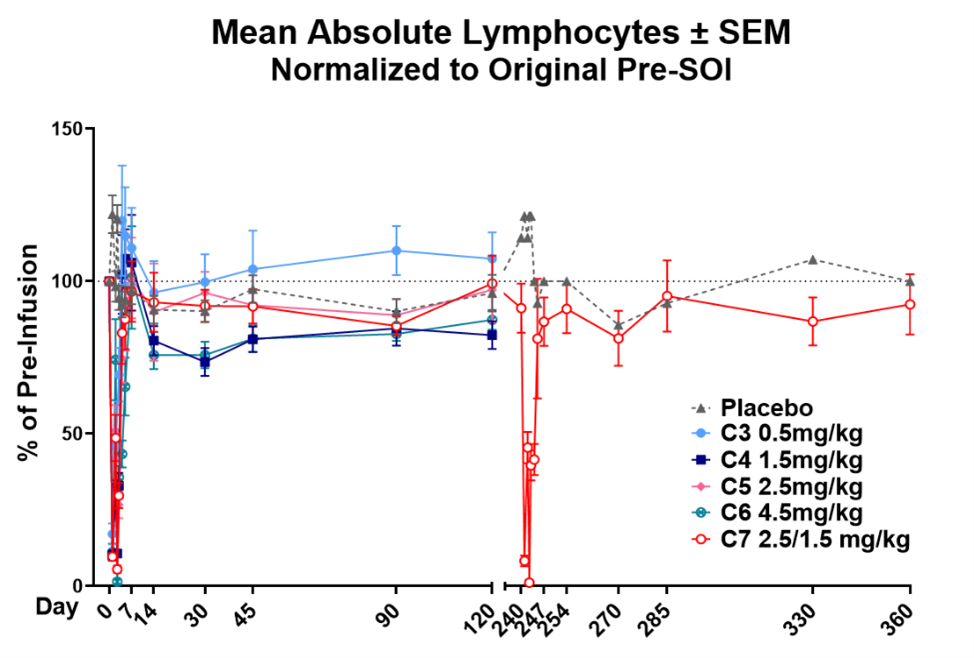
Table 1
“These promising Phase 1 data support our belief that SAB-142 is emerging as a potential best-in-class, redosable treatment for delaying progression of stage 3 T1D. All results have met or exceeded our expectations, allowing us to move swiftly into our registrational Phase 2b SAFGUARD study,” said Alexandra Kropotova, M.D., MBA, Chief Medical Officer, SAB BIO. “I would like to thank the clinical trial participants, their families, the clinicians, and our colleagues at collaborating institutions for their invaluable contributions to our clinical trials. We look forward to sharing updates from the SAFEGUARD trial over the next two years.”
“This investigational therapy has been well-tolerated throughout the Phase 1 study, and we look forward to evaluating its effectiveness in new onset T1D. Novel treatment options that can meaningfully alter the course of disease are urgently needed,” said Michael J. Haller, M.D., Professor and Chief of Pediatric Endocrinology, University of Florida. “I am excited about the prospect for benefit with SAB-142 in patients with T1D, and I look forward to leading the SAFEGUARD Phase 2b trial.”
Based on these data, SAB BIO has advanced SAB-142 into a registrational Phase 2b trial SAFEGUARD to evaluate SAB-142 in adult and pediatric patients with new-onset, stage 3 T1D. The SAFEGUARD trial is enrolling at multiple sites around the world, and the Company is on track to dose the first patient by the end of the year.
About the Phase 1 Trial of SAB-142
The Phase 1 trial of SAB-142 is a randomized, double-blind, placebo-controlled, single-ascending dose, adaptive design clinical study among healthy volunteers and one cohort of participants with T1D. The study objectives include establishing safety, tolerability, pharmacokinetic (PK), immunogenicity, and pharmacodynamic (PD) profile for SAB-142 with a single 0.03-4.5mg/kg dose plus one cohort with an additional 1.5mg/kg dose.
About SAB-142
SAB-142 is a potentially disease-modifying, redosable immunotherapy in clinical development for the treatment of autoimmune type 1 diabetes (T1D). SAB-142 is a multi-specific, fully human anti-thymocyte globulin (hATG) with a mechanism of action analogous to that of rabbit ATG (rATG). rATG has demonstrated in multiple clinical trials the ability to slow disease progression in patients with new- or recent-onset of Stage 3 T1D. SAB-142, like rATG, directly targets multiple immune cells involved in destroying pancreatic beta cells, including modulation of “bad acting” T-lymphocytes like cytotoxic T-cells. By stopping immune cells from attacking beta cells, this treatment has the potential to preserve insulin-producing beta cells.
About SAB BIO
SAB BIO is a clinical-stage biopharmaceutical company focused on developing multi-specific, high-potency, human immunoglobulin G (hIgG) to treat and prevent immune and autoimmune disorders. The Company’s lead candidate, SAB-142, targets autoimmune T1D with a disease-modifying therapeutic approach that aims to change the T1D treatment paradigm by delaying onset and potentially preventing disease progression of Stage 3 T1D patients. Using advanced genetic engineering and antibody science, SAB BIO developed a proprietary technology which holds the potential to generate additional novel therapeutic candidates utilizing the human immune response, without the need for human donors or convalescent plasma. SAB BIO has optimized genetic engineering in the development of transchromosomic cattle, or Tc-Bovine™, to produce hIgG. SAB BIO’s drug development production system is able to generate a diverse repertoire of specifically targeted, high-potency, hIgGs that can address a wide range of serious unmet needs in human diseases. For more information, visit www.sab.bio.
Forward-Looking Statements
Certain statements made in this current report that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “to be,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook,” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding future events, including statements about the development and clinical trial results of the Company’s T1D program and other discovery programs.
These statements are based on the current expectations of SAB BIO and are not predictions of actual performance, and are not intended to serve as, and must not be relied on, by any investor as a guarantee, prediction, definitive statement, or an assurance, of fact or probability. These statements are only current predictions or expectations, and are subject to known and unknown risks, uncertainties and other factors which may be beyond our control. Actual events and circumstances are difficult or impossible to predict, and these risks and uncertainties may cause our or our industry’s results, performance, or achievements to be materially different from those anticipated by these forward-looking statements. A further description of risks and uncertainties can be found in the sections captioned “Risk Factors” in our most recent annual report on Form 10-K, subsequent quarterly reports on Form 10-Q, as may be amended or supplemented from time to time, and other filings with or submissions to, the U.S. Securities and Exchange Commission, which are available at https://www.sec.gov/. Except as otherwise required by law, SAB BIO disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events, or circumstances or otherwise.
CONTACTS
Investor Relations:
Cristi Barnett
ir@sab.bio
Media:
Sheila Carlson
media@sab.bio
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/56c69165-5952-46ec-adb4-af37827914a8








