Positive Clinical Results from Phase 2 Trial of SGX945 for the Treatment of Behçet's Disease Published in Rheumatology (Oxford)
Rhea-AI Summary
Soligenix (Nasdaq: SNGX) announced publication of Phase 2a results for SGX945 (dusquetide) in Behçet's Disease (oral aphthous ulcers) on December 18, 2025. In an open-label pilot (n=8) SGX945 showed beneficial effects for 7 of 8 patients during 4 weeks of treatment and maintained effects through a 4-week follow-up.
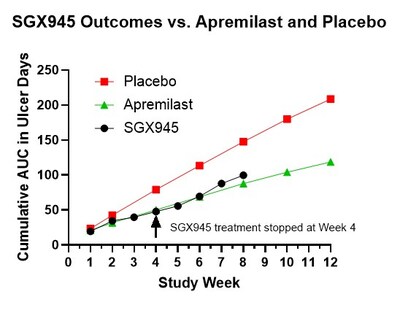
Using the apremilast Phase 3 AUC endpoint, SGX945 showed a 40% improvement at Week 4 versus placebo (apremilast 37%) and a 32% improvement at Week 8 despite treatment stopping at Week 4 (apremilast 41% at Week 8). No treatment-related adverse events reported.
Positive
- 40% AUC improvement at Week 4 versus placebo
- 32% AUC improvement at Week 8 after treatment stopped
- 7 of 8 patients reported clinical benefit
- No treatment-related adverse events reported
Negative
- Very small sample size: n=8 limits statistical confidence
- Open-label design and cross-trial comparison to apremilast limit direct comparability
News Market Reaction
On the day this news was published, SNGX declined NaN%, reflecting a moderate negative market reaction. Argus tracked a peak move of +6.8% during that session. Argus tracked a trough of -2.3% from its starting point during tracking. Our momentum scanner triggered 10 alerts that day, indicating notable trading interest and price volatility. Trading volume was elevated at 2.4x the daily average, suggesting increased selling activity.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
SNGX was down 19.08% with elevated volume, while key biotech peers showed mixed moves; the only momentum-screen peer, XTLB, was down 5.31% without news, suggesting a largely stock-specific reaction.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Dec 05 | Strategic editorial | Positive | -2.4% | Editorial spotlighted rare-disease focus and HyBryte’s late-stage status. |
| Dec 03 | Strategic editorial | Positive | -1.9% | Editorial on HyBryte and rare-disease burden in underserved CTCL space. |
| Nov 19 | Clinical milestone | Positive | -3.6% | FLASH2 Phase 3 CTCL enrollment milestone and stronger-than-expected blinded response. |
| Nov 07 | Earnings update | Neutral | +7.0% | Q3 2025 loss with sufficient cash runway and continued Phase 3 progress. |
| Oct 14 | Management/clinical | Positive | -5.2% | Updated CTCL medical advisory board to support HyBryte FLASH2 strategy. |
Recent history shows frequent negative price reactions following generally positive or strategic updates, with only one of the last five news events seeing an aligned positive move.
Over the last few months, Soligenix has emphasized late-stage rare-disease programs, including HyBryte’s confirmatory Phase 3 CTCL trial and SGX945 for Behçet's Disease. Clinical milestones such as the FLASH2 interim-enrollment update on Nov 19, 2025 and earlier HyBryte safety confirmation have coincided with mixed-to-negative price moves. The Q3 2025 results on Nov 7, 2025 highlighted a $2.5M net loss but adequate cash, and a medical advisory board refresh on Oct 14, 2025 supported CTCL strategy. Today’s Behçet’s publication fits a pattern of clinically constructive news against a pressured share price.
Market Pulse Summary
This announcement highlights published Phase 2a results for SGX945 in Behçet’s Disease, with 7 of 8 patients benefiting and a 40% AUC improvement versus placebo at Week 4, alongside sustained effects to Week 8 and no treatment‑related adverse events. In context with recent HyBryte Phase 3 milestones and Q3 cash of $10.5M, the news adds to Soligenix’s rare‑disease narrative. Investors may watch for follow‑on trial design, reformulation progress, and updated clinical timelines.
Key Terms
phase 2a medical
phase 3 medical
area under the curve (AUC) medical
placebo medical
orphan disease medical
subcutaneous injection medical
innate immune system medical
AI-generated analysis. Not financial advice.
- Study results support advancing SGX945 in this difficult-to-treat orphan disease
- Results suggest potential durability of response for maintenance therapy
The Phase 2a study, evaluating control of oral ulcers in Behçet's Disease, reported beneficial effects for 7 of 8 patients, over the 4 weeks of treatment as well as a potentially enduring effect through the 4 weeks of follow-up. Many Behçet's Disease treatments, including the most recently approved apremilast (Otezla®), do not have an enduring impact, necessitating frequent and continuous administration. Using the Phase 3 study of apremilast as a baseline for comparison, this open-label study indicated that the area under the curve (AUC; a composite measurement of both peak number of oral ulcers and the time to resolution of the oral ulcers), average number of oral ulcers, and improvements in oral pain for SGX945 were similar to outcomes obtained in the apremilast study. Notably, outcomes in weeks 5 through 8 continued to show similar outcomes, even though apremilast treatment was continued through this period whereas SGX945 treatment was stopped at Week 4, per study design.
The primary endpoint in the Phase 3 apremilast study was the AUC of the mean number of ulcers versus time. Using this same endpoint after 4 weeks of treatment, the SGX945 treated group had a
The improvements in oral pain mimicked the results in the AUC measurement. Seven of 8 patients reported perceived benefit with SGX945 treatment, with common outcomes including reduced duration of oral ulcers, reduced number of oral ulcers, and reduced oral pain. One patient began the study with a punctuated skin ulcer and this also resolved during the 4-week treatment with SGX945. Skin ulcers are generally considered very difficult to resolve and usually require protracted treatment. Notably, some patients also explicitly reported experiencing fewer ulcers and less pain during the 4-week follow-up period, as also reflected in the numerical analysis. SGX945 was well-tolerated with no treatment-related adverse events. Common adverse events for apremilast included diarrhea (
"We are pleased to publish the data from our recent SGX945 Phase 2a trial in aphthous ulcers of Behçet's Disease, enabling the medical community to evaluate this novel mechanism and therapeutic," stated Christopher J. Schaber, PhD, President and Chief Executive Officer of Soligenix. "Given the role of the innate immune system, we believe that dusquetide may offer significant relief to patients. With these, now published, results, we intend to embark on a reformulation of SGX945 to enable home-based treatment, using subcutaneous injection as used for example with weight-loss drugs. We are excited to expand dusquetide's development into different innate immune-related inflammatory conditions, such as Behçet's Disease, as a component of our long-term strategy to enhance the value of this unique compound. Behçet's Disease is an area of unmet medical need, with up to 18,000 people in the
About Dusquetide
Dusquetide, the active ingredient in SGX945 (Behçet's Disease) and SGX942 (oral mucositis), is an innate defense regulator (IDR), a new class of short, synthetic peptides. It has a novel mechanism of action whereby it modulates the body's reaction to both injury and infection towards an anti-inflammatory, anti-infective, and tissue healing response. IDRs have no direct antibiotic activity but, by modulating the host's innate immune system responses, increase survival after infections caused by a broad range of Gram-negative and Gram-positive bacterial pathogens. Dusquetide also accelerates resolution of tissue damage following exposure to a variety of agents including bacterial pathogens, trauma, and chemo- and/or radiation therapy. Preclinical efficacy and safety have been demonstrated in numerous animal disease models including mucositis, colitis, macrophage activation syndrome as well as bacterial infections. In addition, potential anti-tumor activity has been demonstrated in multiple in vitro and in vivo xenograft studies.
Dusquetide has demonstrated safety and tolerability in a Phase 1 clinical study in 84 healthy human volunteers. In Phase 2 and 3 clinical studies with dusquetide in over 350 subjects with oral mucositis due to chemoradiation therapy for head and neck cancer, positive efficacy results were demonstrated, including potential long-term ancillary benefits.
Dusquetide has also demonstrated biological efficacy and safety in a Phase 2a pilot study in 8 patients with Behçet's Disease. The Phase 2a study was an open-label study designed to be highly comparable (e.g., study endpoints, inclusion-exclusion criteria) to the published Phase 3 study which was used to support marketing approval of apremilast (Otezla®) for oral ulcers in Behçet's disease. The primary endpoint in the Phase 3 apremilast study was the area under the curve (AUC) of the mean number of ulcers versus time. Using this same endpoint after 4 weeks of treatment, the SGX945 treated group had a
Soligenix has a strong intellectual property position in the IDR technology platform, including composition of matter for dusquetide and related analogs. Dusquetide was developed pursuant to discoveries made by Professors B. Brett Finlay, PhD and Robert Hancock, PhD of the University of
About Behçet's Disease
Behçet's Disease is commonly known as an inflammatory disorder of the blood vessels (vasculitis). Often first diagnosed in young adults, its effects and severity will wax and wane over time. Major signs and symptoms usually include mouth sores (approximately
Behçet's Disease is thought to be an auto-immune disease with both genetic and environmental factors. It is most common along the "Silk Road" in the
There is no cure for Behçet's Disease, rather treatments are prescribed to manage symptoms. Treatments may include both maintenance therapies and those specifically addressing flares (e.g., mouth ulcers, genital ulcers and leg ulcers). Corticosteroids are generally applied topically to sores and as eyedrops and may also be given systemically to reduce inflammation. Although used frequently, they have limited efficacy over the long-term and have significant side effects that become more concerning with more chronic use. Genital ulcers are often associated with significant genital scarring while leg ulcers can result in a post-thrombotic syndrome. Other treatments for Behçet's Disease flares involve suppressing the immune system with drugs (e.g., cyclosporine or cyclophosphamide). These drugs come with a higher risk of infection, liver and kidney problems, low blood counts and high blood pressure. Finally, anti-inflammatory drugs are also used, including anti-TNF medications. The only approved drug in Behçet's Disease is apremilast, which is used as a maintenance therapy to prevent formation of oral ulcers. Unfortunately, apremilast must be used continuously to be effective and is associated with both high cost and side effects including diarrhea, nausea, upper respiratory tract infection and headache.
About Soligenix, Inc.
Soligenix is a late-stage biopharmaceutical company focused on developing and commercializing products to treat rare diseases where there is an unmet medical need. Our Specialized BioTherapeutics business segment is developing and moving toward potential commercialization of HyBryte™ (SGX301 or synthetic hypericin sodium) as a novel photodynamic therapy utilizing safe visible light for the treatment of cutaneous T-cell lymphoma (CTCL). With successful completion of the second Phase 3 study, regulatory approvals will be sought to support potential commercialization worldwide. Development programs in this business segment also include expansion of synthetic hypericin (SGX302) into psoriasis, our first-in-class innate defense regulator (IDR) technology, dusquetide (SGX942) for the treatment of inflammatory diseases, including oral mucositis in head and neck cancer, and (SGX945) in Behçet's Disease.
Our Public Health Solutions business segment includes development programs for RiVax®, our ricin toxin vaccine candidate, as well as our vaccine programs targeting filoviruses (such as Marburg and Ebola) and CiVax™, our vaccine candidate for the prevention of COVID-19 (caused by SARS-CoV-2). The development of our vaccine programs incorporates the use of our proprietary heat stabilization platform technology, known as ThermoVax®. To date, this business segment has been supported with government grant and contract funding from the National Institute of Allergy and Infectious Diseases (NIAID), the Defense Threat Reduction Agency (DTRA) and the Biomedical Advanced Research and Development Authority (BARDA).
For further information regarding Soligenix, Inc., please visit the Company's website at https://www.soligenix.com and follow us on LinkedIn and Twitter at @Soligenix_Inc.
This press release may contain forward-looking statements that reflect Soligenix's current expectations about its future results, performance, prospects and opportunities, including but not limited to, potential market sizes, patient populations and clinical trial enrollment. Statements that are not historical facts, such as "anticipates," "estimates," "believes," "hopes," "intends," "plans," "expects," "goal," "may," "suggest," "will," "potential," or similar expressions, are forward-looking statements. These statements are subject to a number of risks, uncertainties and other factors that could cause actual events or results in future periods to differ materially from what is expressed in, or implied by, these statements. Soligenix cannot assure you that it will be able to successfully develop, achieve regulatory approval for or commercialize products based on its technologies, particularly in light of the significant uncertainty inherent in developing therapeutics and vaccines against bioterror threats, conducting preclinical and clinical trials of therapeutics and vaccines, obtaining regulatory approvals and manufacturing therapeutics and vaccines, that product development and commercialization efforts will not be reduced or discontinued due to difficulties or delays in clinical trials or due to lack of progress or positive results from research and development efforts, that it will be able to successfully obtain any further funding to support product development and commercialization efforts, including grants and awards, maintain its existing grants which are subject to performance requirements, enter into any biodefense procurement contracts with the
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/positive-clinical-results-from-phase-2-trial-of-sgx945-for-the-treatment-of-behcets-disease-published-in-rheumatology-oxford-302645452.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/positive-clinical-results-from-phase-2-trial-of-sgx945-for-the-treatment-of-behcets-disease-published-in-rheumatology-oxford-302645452.html
SOURCE SOLIGENIX, INC.