Theralase Clinical Data Presented at Canadian Bladder Cancer Forum and American Urological Association
Rhea-AI Summary
Positive
- 62.5% of patients achieved Complete Response, with 45% maintaining it for 12+ months
- 100% of patients experienced no treatment-related Serious Adverse Events
- One patient demonstrated sustained Complete Response for over 7 years
- Multiple immune checkpoint blocking capabilities demonstrated by RuvidarTM technology
- Enrollment completion expected by mid-2025 with regulatory submissions planned for late 2026
Negative
- Regulatory approval still pending and not guaranteed
- 15 Serious Adverse Events reported, though deemed unrelated to treatment
- 55% of Complete Response patients did not maintain response for 12 months
News Market Reaction
On the day this news was published, TLTFF gained 2.32%, reflecting a moderate positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
Toronto, Ontario--(Newsfile Corp. - May 5, 2025) - Theralase® Technologies Inc. (TSXV: TLT) (OTCQB: TLTFF) ("Theralase®" or the "Company"), a clinical stage pharmaceutical company dedicated to the research and development of small molecules and their formulations, able to be activated by light, radiation, sound and/or other drugs, intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that its latest interim clinical data was recently presented at the Canadian Bladder Cancer Forum 2025 and the American Urological Association 2025 Annual Meeting.
The interim clinical data from Theralase®'s multicenter Phase II Bacillus Calmette-Guérin ("BCG")-Unresponsive Non-Muscle Invasive Bladder Cancer ("NMIBC") Carcinoma In-Situ ("CIS") study ("Study II") was presented by Dr. Girish Kulkarni in podium presentations. Dr. Kulkarni, M.D., Ph.D., FRCSC is a urologic surgeon in the Department of Surgical Oncology, Princess Margaret Cancer Centre, University Health Network, a professor in the Department of Surgery at the University of Toronto and the lead principal investigator in the Theralase® study.
The interim clinical data presented represents world-class safety and efficacy in the treatment of BCG-Unresponsive NMIBC CIS with a light-activated small molecule. Numerous patients have demonstrated durations of response of 3 years or greater, with 1 patient demonstrating a duration of response of over 7 years, after a single treatment.
Study II Interim Clinical Data1:
Theralase® has enrolled and treated 79 patients in Study II, who have been provided the primary Study Procedure (therapeutic dose of Theralase®'s lead small molecule RuvidarTM light-activated by the TLC-3200 medical laser system), with completion of enrollment expected by mid-2025.
Performance to Primary Objective:
For the primary endpoint,
Including patients, who demonstrated an Indeterminate Response ("IR") (negative cystoscopy and positive or suspicious urine cytology), the Total Response increases to
| Primary Endpoint Performance (CR at any Point in Time) | |||
| # | % | Confidence Interval ( | |
| Complete Response ("CR") | 40 | [43.1, 81.9] | |
| Total Response (CR and IR) | 44 | [48.5, 89.1] | |
Performance to Secondary Objective:
For the secondary endpoint,
| Secondary Endpoint Performance (Duration of CR) (450 Days) | |||
| # | % | Confidence Interval ( | |
| Complete Response ("CR") | 18 | [24.2, 65.8] | |
Performance to Tertiary Objective:
For the tertiary endpoint,
| Tertiary Endpoint Performance (Safety) (450 Days) | ||
| # | % | |
| Safety | 64 | |
Patient Response Chart:
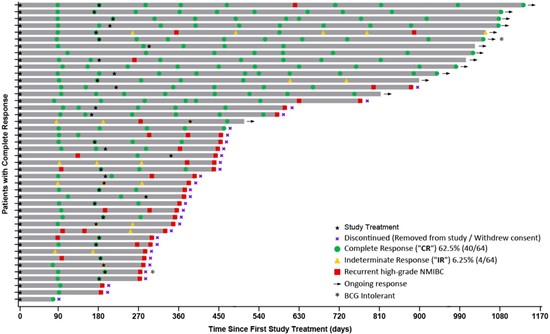
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/2786/250699_theraleseimg1.jpg
The Swimmer's plot is a graphical representation of the interim clinical results (n=64) for patients who achieved a CR at any point in time and their response up to and including 1170 days.
As can be seen in the plot, certain patients (indicated by arrows), who have demonstrated an initial CR at 90 days continue to demonstrate a duration of that response for 3 years or more.
Kaplan-Meier Curve:
The Kaplan-Meier ("KM") Curve illustrates graphically, for patients who have achieved a CR, the duration of CR and probability of that CR continuing in the future, when all clinical data of the Study II is analyzed.

To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/2786/250699_5394f321d4e56baf_002full.jpg
According to the KM Curve, if CR is obtained, then the patient has a ≥
Serious Adverse Events
For 79 patients treated in Study II, there have been 15 Serious Adverse Events ("SAEs") reported:
- 1 - Grade 1 (resolved within 9 days)
- 3 - Grade 2 (resolved within 1, 1 and 33 days, respectively)
- 7 - Grade 3 (resolved within 1, 2, 3, 4, 4, 82 and unknown days, respectively)
- 3 - Grade 4 (resolved within 3, 6 and 8 days, respectively)
- 1 - Grade 5
Theralase® believes all SAEs reported to date are unrelated to the Study II Drug or Study II Device.
Pending regulatory approval, this innovative technology represents a significant opportunity for an advancement in bladder cancer treatment, with only one treatment, by providing a safe and effective therapy for patients, who have exhausted their standard of care therapeutic options and are facing radical cystectomy (bladder removal).
Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer, Theralase® stated, "I am delighted that the interim clinical data of Study II continues to demonstrate both high safety and high efficacy in the treatment of patients diagnosed with BCG-Unresponsive NMIBC CIS. Management of BCG-Unresponsive NMIBC CIS is and remains an unmet need and I look forward to the completion of enrollment in the clinical study this summer, which pending regulatory approval could provide an opportunity for patients to receive this treatment as part of routine clinical practice in both Canada and the United States. What we have learned in our research is that Theralase®'s light-activated small molecule, Ruvidar®, is able to destroy cancer through the production of reactive oxygen species and stimulation of the immune system by blocking multiple immune checkpoints, such as: CD474 (a dominant macrophage checkpoint signal on cancer cells that signals the immune system whether to destroy them or leave them alone), PD-L15 (a protein that acts as a "brake" on the immune system, preventing T cells from attacking cancer cells) and the recently discovered reduction of deubiquitinating enzymes6 (an important class of enzymes, which have been linked to numerous cancers and neurogenerative diseases)."
Roger DuMoulin-White, B.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer, Theralase® stated, "The presentation of the interim clinical data to the urological community at the major Canadian and American conferences provides the global urologist community and healthcare professionals, an opportunity to see firsthand the strong clinical data that the uro-oncologists involved in the clinical study have been able to deliver with the Theralase® technology. RuvidarTM has proven to be a very versatile small molecule, with its ability to destroy cancer, bacteria and viruses, when activated by light, radiation, sound or even other drugs. Pending regulatory approval, the latest clinical data presented supports the use of light-activated Ruvidar™ by the urology community to safely and effectively treat patients inflicted with BCG-Unresponsive NMIBC CIS, helping to revolutionize the treatment landscape for bladder cancer. As Theralase® wraps up the Phase II NMIBC clinical study in 2025 with a Health Canada and FDA regulatory submission planned in late 2026, I look forward to working with our world-class scientists, researchers and medical doctors in the commencement of numerous new clinical studies, focused on hard to treat viral infections, such as herpes simplex virus lesions (cold sores), through to some of the deadliest and most difficult to treat cancers in the world, such as: glioblastoma multiforme, non-small cell lung cancer, pancreatic cancer, leukemia, muscle invasive bladder cancer and colorectal cancer."
About Carcinoma In-Situ:
CIS of the bladder is an aggressive type of NMIBC characterized as a flat, high-grade tumour confined to the urothelial layer. NMIBC comprises approximately
Management of CIS of the bladder remains a complex and challenging endeavor due to its high rate of recurrence and progression. Although it is typically grouped with other NMIBCs, its higher grade and aggressiveness make it a unique clinical entity. Intravesical BCG is the standard first-line treatment given its superiority to other agents; however, high rates of BCG failure highlight the need for additional therapies.8
CIS in the bladder is associated with a less favourable prognosis. It is more likely to recur after treatment. There is also a greater risk of CIS developing into Muscle Invasive Bladder Cancer ("MIBC").9
References:
1 Theralase(R) Releases 2024 Annual Financial Statements
2 A Phase 1b Clinical Study of Intravesical Photodynamic Therapy in Patients with Bacillus Calmette-Guérin-unresponsive Non-muscle-invasive Bladder Cancer - ScienceDirect
3 Ruvidar Demonstrates 7 Year Complete Response
4 Theralase(R) Files US Patent for Enhanced Immunotherapy
5 Ruvidar(TM) Enhances Efficacy of Cancer Drug
6 Theralase Discovers New Mechanism of Action of Lead Drug
7 Llano A, Chan A, Kuk C, Kassouf W, Zlotta AR. Carcinoma In Situ (CIS): Is There a Difference in Efficacy between Various BCG Strains? A Comprehensive Review of the Literature. Cancers (Basel). 2024 Jan 5;16(2):245. doi: 10.3390/cancers16020245. PMID: 38254736; PMCID: PMC10813486.
8 Tang DH, Chang SS. Management of carcinoma in situ of the bladder: best practice and recent developments. Ther Adv Urol. 2015 Dec;7(6):351-64. doi: 10.1177/1756287215599694. PMID: 26622320; PMCID: PMC4647140.
9 Prognosis and survival for bladder cancer | Canadian Cancer Society
About the Canadian Bladder Cancer Forum
The Canadian Urological Association Office of Education partnered with Bladder Cancer Canada, Dr. Peter Black (Chair) and Dr. Kala Sridhar (Vice-Chair) of Bladder Cancer Canada's Medical Advisory and Research Board to organize an important educational event dedicated to the research and treatment of bladder cancer. The meeting hosted over 60 participants from a multi-disciplinary setting, with urologists, medical oncologists, radiation oncologists, clinicians, scientists, researchers and patients, in a live setting in Toronto, Ontario.
About the American Urological Association
The American Urological Association ("AUA") is a professional association in the United States for urology professionals. It is headquartered at the William P. Didusch Center for Urologic History in Maryland. AUA works with many international organizations, representing urologists from across the world. The 2025 event in Las Vegas, Nevada hosted over 10,000 urologists and healthcare professionals.
About Study II:
Study II utilizes the therapeutic dose of the patented Study Drug (RuvidarTM) (0.70 mg/cm2) activated by the proprietary Study Device (TLC-3200 Medical Laser System). Study II is focused on enrolling and treating approximately 90 BCG-Unresponsive NMIBC CIS patients in 14 clinical study sites located in Canada and the United States.
About Ruvidar™
Ruvidar™ (TLD-1433) is a small molecule, able to be activated by light, radiation, sound and/or other drugs, intended for the safe and effective destruction of various cancers, bacteria and viruses.
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecule compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedarplus.ca.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-Looking Statements:
This news release contains Forward-Looking Statements ("FLS") within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company's proposed development plans with respect to small molecules and their drug formulations. FLS may be identified by the use of the words "may, "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of the Company's management regarding future research, development and commercialization of the Company's small molecules; their drug formulations; preclinical research; clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete various clinical studies in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its small molecule and drug formulations; the risk that access to sufficient capital to fund the Company's operations may not be available on terms that are commercially favorable to the Company or at all; the risk that the Company's small molecule and drug formulations may not be effective against the diseases tested in its clinical studies; the risk that the Company fails to comply with the terms of license agreements with third parties and as a result loses the right to use key intellectual property in its business; the Company's ability to protect its intellectual property; the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these FLS, which are not a guarantee of future performance. There can be no assurance that FLS will prove to be accurate as such FLS involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the FLS.
Although the FLS contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these FLS.
All FLS are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such FLS.
For investor information on the Company, please feel to reach out Investor Inquiries - Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer X 224
khachey@theralase.com

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/250699







