Theralase Provides Corporate Update
Rhea-AI Summary
Positive
- 91% enrollment completion in Study II with strong interim results (62.3% Complete Response rate)
- 100% safety profile with no serious adverse events related to Study Drug or Device
- Demonstrated long-term efficacy with some patients maintaining Complete Response up to 7 years
- FDA impressed with interim clinical data achieved with single treatment
- Ruvidar demonstrates 10-year shelf life stability
- Multiple pipeline developments in GBM, NSCLC, MIBC, and HSV-1 advancing to Phase I/II trials
- Successfully raised CAD 6.3 million in past 2 years
Negative
- NDA submission and potential approval timeline extends to Q4 2026/Q1 2027
- Company decided not to pursue Break Through Designation with FDA
- Multiple early-stage programs will require significant additional funding and development time
News Market Reaction 1 Alert
On the day this news was published, TLTFF declined 0.54%, reflecting a mild negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
Toronto, Ontario--(Newsfile Corp. - May 20, 2025) - Theralase® Technologies Inc. (TSXV: TLT) (OTCQB: TLTFF) ("Theralase®" or the "Company"), a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecules and their formulations, intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to provide a corporate update outlining the Company's strategic objectives.
1) Bacillus Calmette-Guérin ("BCG")-Unresponsive Non-Muscle Invasive Bladder Cancer ("NMIBC") Carcinoma In-Situ ("CIS") Registrational Clinical Study ("Study II")
Theralase® has made steady progress on the completion of Study II by enrolling and providing the primary study procedure for 82 patients out of a target of 90 patients (
According to the clinical study design, a patient is considered to have completed Study II, if they receive the study procedure (study drug activated by study device) and have been assessed by the Principal Investigator ("PI") for up to 15 months or they have been prematurely removed from the clinical study by the PI for failure to respond or failure to comply with the clinical study design.
According to this definition, 69 patients have completed Study II (with 13 patients on study with pending clinical data) resulting in the following interim clinical data in support of the Study II endpoints.
| Primary Endpoint Performance (CR at any Point in Time) | |||
| # | % | Confidence Interval ( | |
| Complete Response ("CR") | 43/69 | [43.7, 80.9] | |
| Total Response (CR and IR) | 48/69 | [49.9, 89.2] | |
| Secondary Endpoint Performance (Duration of CR) (15 months) | |||
| # | % | Confidence Interval ( | |
| Complete Response ("CR") | 18/43 | [22.5, 61.2] | |
| Tertiary Endpoint Performance (Safety) (15 months) | ||
| # | % | |
| Safety | 69/69 | |
For 82 patients treated in Study II, there have been no Serious Adverse Events ("SAEs") directly related to the Study Drug or Study Device.
Outside of the defined endpoints of Study II, Theralase® has demonstrated a duration of CR at extended time points, as follows:
| Duration of CR | |||
| Time | # | % | Confidence Interval ( |
| 2 Years | 10/43 | [8.8, 37.7] | |
| 3 Years | 9/43 | [7.3, 34.6] | |
| 7 Years | 1/43 | [0.0, 6.9] | |
Note: Not all patients have been assessed at these extended time points. As more clinical data is collected, the duration of CR at 2 and 3 years may increase.
If approved by the regulatory authorities, the interim clinical data obtained could significantly benefit patients who are faced with a radical cystectomy (removal of their bladder), as the Theralase® treatment provides a strong initial CR and an equally strong duration of that CR over time. It is made even more impressive by the fact that this clinical data was achieved with only one study procedure in the majority of cases.
Theralase® is on track to complete enrollment in Study II by the summer of 2025.
This will allow the Company to report on 75 patients who have completed Study II in December 2025 and to report on all 90 patients by September 2026.
Upon follow-up of all patients, the Company plans to submit a New Drug Application ("NDA") to Health Canada and the FDA in 4Q2026, with a decision expected by the respective regulatory authorities on a marketing approval by 1Q2027.
As Theralase® completes enrollment in Study II, it is actively searching for commercialization partners for international marketing and sales of Ruvidar®.
To this end, Theralase® is in various stages of initial and advanced discussions with international pharmaceutical companies for various geographical territories concerning:
- Licensing of the light-activated Ruvidar® for BCG-Unresponsive NMIBC CIS
- Collaborative research focused on investigating light-activated Ruvidar® in the treatment of NMIBC
- Collaborative research focused on combining Ruvidar® with other FDA approved drugs
In recent discussions with the FDA, the Company has decided that since Study II is
Ruvidar® has demonstrated 10 years shelf life, strongly supporting the stability of the molecule and the ability of clinics to store the small molecule for extended periods of time.
2) Glio Blastoma Multiforme ("GBM") Brain Cancer Treatment
Theralase® has successfully completed pre-clinical research to develop Ruvidar® for the destruction of GBM. The Company expects to complete Good Laboratory Practice ("GLP") toxicology in 4Q2025 to allow commencement of a Phase I/II adaptive clinical study in 1Q2026.
As an example of the effectiveness of Rutherrin® (Intra Venous ("IV") formulation of Ruvidar®) activated by radiation in the destruction of brain cancer, refer to Figure 1.

Figure 1: Destruction of human glioma cells treated with radiation-activated Rutherrin® versus radiation alone
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/2786/252699_eaed8a0274082a0f_001full.jpg
3) Non-Small Cell Lung Cancer ("NSCLC") Treatment
Theralase® has finished conducting pre-clinical research to develop Ruvidar® for the destruction of NSCLC. Mice treated with x-ray activated Rutherrin® in a Lewis Lung Cancer ("LLC1") orthotopic model have demonstrated up to a 4-fold slower tumour progression, based on the Magnetic Resonance Imaging assessment of tumour volumes.

Figure 2: Tumour volume analysis in mice after tumour inoculation and treatment with either radiation alone or a combined treatment of Rutherrin ® and radiation treatment
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/2786/252699_eaed8a0274082a0f_002full.jpg
As shown in Figure 2, there is a significant delay in tumour progression in mice treated with x-ray activated Rutherrin® versus radiation alone (p> 0.001). Mice treated with x-ray activated Rutherrin® demonstrate that the tumour is regressing over time.
The Company expects to complete GLP toxicology in 4Q2025 to allow commencement of a Phase I/II adaptive clinical study in 1Q2026.
4) Muscle Invasive Bladder Cancer ("MIBC") Treatment
Theralase® is conducting pre-clinical research to develop Ruvidar® for the destruction of MIBC as an intravenous treatment for patients that are inflicted with this disease. The Company expects to complete GLP toxicology in 4Q2025 to allow commencement of a Phase I/II adaptive clinical study in 1Q2026.

Figure 3: Full depth tumour necrosis also occurs in muscle invasive bladder tumours, with no damage to healthy muscle tissue (Light-activated Ruvidar®)
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/2786/252699_theralasefigure3.jpg
5) Leukemia, Lymphoma and Myeloma Treatment
Theralase® is conducting pre-clinical research to develop Ruvidar® for the destruction of Leukemia, Lymphoma and Myeloma as an extracorporeal treatment for patients that are inflicted with these diseases. Theralase® plans to develop this technology in 2026.
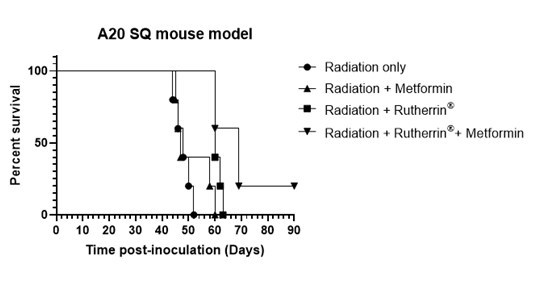
Figure 7. Evaluation of Leukemia in a mouse model
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/2786/252699_eaed8a0274082a0f_006full.jpg
6) Herpes Simplex Virus ("HSV-1") Topical Treatment for Cold Sore Lesions
Theralase® is conducting pre-clinical research to develop Ruvidar® for the inactivation of HSV-1 as a topical treatment for the billions of patients worldwide that are inflicted with cold sores on an annual basis. The Company is using its internal research laboratory to develop the topical formulation to be used in preclinical and clinical evaluation.
The Company expects to complete GLP toxicology in 4Q2025 to allow commencement of a Phase I/II adaptive clinical study in 1Q2026.
The research, which demonstrated that RuvidarTM was effective at inactivating both enveloped and non-enveloped viruses, alone and when light activated was accepted in a peer-reviewed publication, Heliyon, and can be reviewed at https://doi.org/10.1016/j.heliyon.2024.e32140
An example of the efficacy of Ruvidar® versus standard of care therapies (i.e.: Acyclovir or Abreva) is demonstrated below:

Figure 4. Acyclovir (
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/2786/252699_eaed8a0274082a0f_007full.jpg

Figure 5. Abreva (
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/2786/252699_eaed8a0274082a0f_008full.jpg

Figure 6. RuvidarTM (
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/2786/252699_eaed8a0274082a0f_009full.jpg
7) Cross Listing to a US Exchange
The Company has raised $CAN 6.3 million over the last 2 years in support of it research and development programs. It is currently investigating the use of a full-service investment bank in the United States to advise on potential financings and US listing opportunities. Information on any future financings will be released once available in accordance with applicable securities laws.
2025/2026: Potentially Transformative Years
Theralase® is positioning itself to deliver on a series of significant clinical and corporate milestones in 2025; including:
- Completing Study II and submitting an NDA to Health Canada and the FDA
- Commencing Phase I/II adaptive clinical studies in: GBM, NSCLC, MIBC, HSV and Leukemia
- Evaluating strategic financing and listing opportunities in the US market
These efforts should collectively support long-term shareholder value and reinforce Theralase®'s leadership in light / radiation-activated drug therapy for the destruction of various cancers and viruses.
About Study II:
Study II utilizes the therapeutic dose of the patented study drug (Ruvidar®) (0.70 mg/cm2) activated by the proprietary study device (TLC-3200 Medical Laser System). Study II is focused on enrolling and treating approximately 90 BCG-Unresponsive NMIBC CIS patients in 12 clinical study sites located in Canada and the United States.
About Ruvidar®:
Ruvidar®(TLD-1433) is a small molecule, able to be activated by light, radiation, sound and/or other drugs, intended for the safe and effective destruction of various cancers, bacteria and viruses.
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecule compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedarplus.ca.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements:
This news release contains Forward-Looking Statements ("FLS") within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company's proposed development plans with respect to small molecules and their drug formulations. FLS may be identified by the use of the words "may, "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of the Company's management regarding future research, development and commercialization of the Company's small molecules; their drug formulations; preclinical research; clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete various clinical studies in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its small molecule and drug formulations; the risk that access to sufficient capital to fund the Company's operations may not be available on terms that are commercially favorable to the Company or at all; the risk that the Company's small molecule and drug formulations may not be effective against the diseases tested in its clinical studies; the risk that the Company fails to comply with the terms of license agreements with third parties and as a result loses the right to use key intellectual property in its business; the Company's ability to protect its intellectual property; the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these FLS, which are not a guarantee of future performance. There can be no assurance that FLS will prove to be accurate as such FLS involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the FLS.
Although the FLS contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these FLS.
All FLS are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such FLS.
For investor information on the Company, please feel to reach out Investor Inquiries - Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer X 224
khachey@theralase.com

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/252699








