Vivos Therapeutics Receives Medicare Approval for VidaSleep™ Oral Appliance
Rhea-AI Summary
Vivos Therapeutics (NASDAQ: VVOS) has achieved a significant milestone as its VidaSleep™ oral appliance received approval from the Centers for Medicare & Medicaid Services (CMS) for treating mild to moderate obstructive sleep apnea (OSA) and snoring in adults.
The VidaSleep™ device, featuring Vivos' patented and FDA-cleared Unilateral Bite Block technology, joins the company's mmRNA® device on the PDAC list of covered oral appliances. This dual-PDAC approval makes Vivos the only company with two Medicare-covered oral appliances, expanding access to millions of Medicare beneficiaries.
With an estimated 80 million Americans affected by OSA and over 80% of cases undiagnosed, Vivos is targeting the $36 billion sleep therapy market. The VidaSleep™ device is designed to offer a cost-effective solution without compromising efficacy, maintaining strong gross margins while expanding accessibility through Medicare and commercial insurance networks.
Positive
- Medicare approval enables access for millions of beneficiaries
- Only company with two Medicare-covered oral appliances
- Targets $36 billion sleep therapy market opportunity
- Maintains strong gross profit margins despite cost-effective pricing
- Approval likely to influence coverage by private insurers
- Device can be used as standalone therapy or with CPAP
Negative
- Enters a market with 80% of cases currently undiagnosed
- Faces competition from established CPAP machines and surgical options
News Market Reaction
On the day this news was published, VVOS gained 22.47%, reflecting a significant positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
Important Milestone Clears the Way for Millions of Medicare Beneficiaries with Sleep Apnea to Access Vivos’ Patented and FDA-Cleared Oral Appliance Treatment
LITTLETON, Colo., July 01, 2025 (GLOBE NEWSWIRE) -- Vivos Therapeutics, Inc. (NASDAQ: VVOS) (“Vivos” or the “Company”), a pioneering medical technology company revolutionizing the treatment of obstructive sleep apnea (OSA) and snoring, today announced that its VidaSleep™ oral appliance, featuring Vivos’ patented and U.S. Food and Drug Administration (FDA) cleared Unilateral Bite Block technology, has been approved by the Centers for Medicare & Medicaid Services (CMS) Pricing, Data Analysis and Coding (PDAC) contractor for the treatment of mild to moderate OSA and snoring in adults.
The VidaSleep™ Device
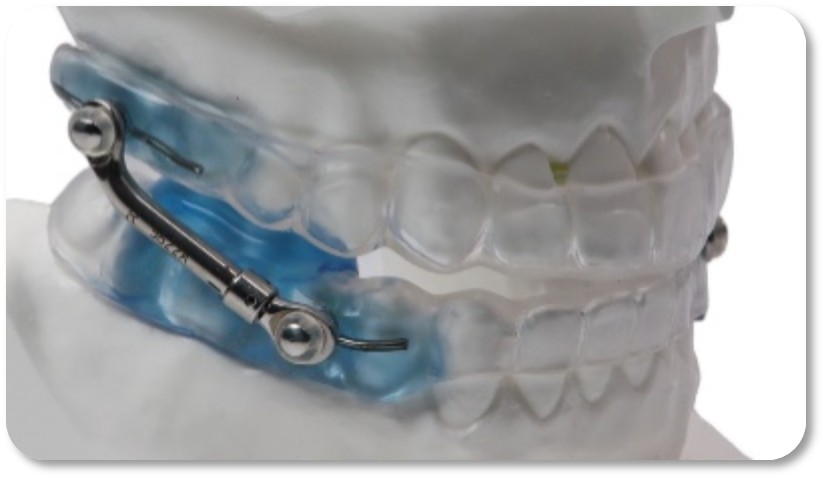
With over
With this approval, VidaSleep™ joins the Vivos mmRNA® on the PDAC list of covered oral appliances, unlocking access for millions of Medicare beneficiaries seeking clinically advanced alternatives to CPAP machines or invasive surgeries. The dual-PDAC approval positions Vivos as the only company with two Medicare-covered oral appliances featuring differentiated and patented technologies designed to address the root causes of OSA while offering patients two distinct pathways and price points for therapy. The VidaSleep™ oral appliance features Vivos’ proprietary Unilateral Bite Block technology, while the mmRNA appliance is part of the FDA-cleared Vivos CARE product line for moderate to severe OSA cases in adults and children.
“The PDAC approval of VidaSleep is another milestone achievement for Vivos, strategically positioning us to significantly augment our presence in the value-based care segment of the sleep apnea market,” said Kirk Huntsman, CEO and Chairman of Vivos Therapeutics. “While mmRNA® remains our premium solution for complex cases, VidaSleep delivers positive clinical outcomes through an optimized design that maximizes accessibility—proving that 'cost-effective' doesn't mean 'compromise.' This dual-device approach allows us to serve every tier of the estimated
Commercial & Clinical Advantages
PDAC approval not only benefits Medicare patients but also incentivizes private insurers to cover Vivos’ appliances. The VidaSleep™ device, like Vivos’ mmRNA® device, is designed for use as a standalone therapy or adjunct to CPAP, offering flexibility for providers and patients. VidaSleep™ offers a compelling value proposition, combining the clinical advantages of Vivos’ proprietary technology with a cost-effective price point designed to expand accessibility. Its streamlined design and efficient manufacturing process allow Vivos to deliver high-impact therapy at a budget-conscious rate—without compromising efficacy or quality—increasing opportunities for broader adoption across Medicare and commercial insurance networks while preserving strong gross margins for the healthcare providers nationwide who can now deliver these solutions with greater confidence in insurance reimbursement.
Sleep Apnea: A Silent Crisis
OSA is linked to severe comorbidities, including heart disease, stroke, and dementia, yet remains grossly underdiagnosed. Vivos’ oral appliances represent a paradigm shift by addressing the structural root causes of OSA, with peer-reviewed studies demonstrating significant improvements in airway patency and symptom resolution, including the recently announced landmark study published in the European Journal of Pediatrics.
About Vivos Therapeutics
Vivos Therapeutics, Inc. (NASDAQ: VVOS) is a medical technology company focused on developing and commercializing innovative diagnostic and treatment methods that promote sleep wellness and health for patients suffering from breathing and sleep issues such as obstructive sleep apnea (OSA) and snoring in adults. Vivos’ Complete Airway Repositioning and/or Expansion (CARE) devices are the only oral appliances cleared by the U.S. Food and Drug Administration (FDA) for adult patients diagnosed with all severity levels of OSA (including severe OSA) and moderate-to-severe OSA in children ages 6 to 17 within the FDA cleared usage for such devices.
Obstructive sleep apnea (OSA) affects over 1 billion people worldwide, yet
Vivos Therapeutics, founded in 2016 and based in Littleton, CO, is changing this. Through innovative technology, education, and collaborations with or acquisitions of functional medicine doctors, and sleep specialists, Vivos is empowering healthcare providers to more thoroughly address the complex needs of patients suffering with OSA.
Vivos’ portfolio of cutting-edge oral appliances offer a proprietary, clinically effective OSA solution that is nonsurgical, noninvasive, and nonpharmaceutical, providing hope to allow patients to Breathe New Life. For more information, visit www.vivos.com.
Cautionary Note Regarding Forward-Looking Statements
This press release, including statements of the Company’s management and other parties made herein, contain “forward-looking statements” (as defined in Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended) concerning future events. Words such as “may”, “would”, “should”, “expects”, “projects”, “opportunity”, “potential,” “intends”, “plans”, “believes”, “anticipates”, “hopes”, “estimates”, “goal”, “aim” and variations of such words and similar expressions are intended to identify forward-looking statements. In this press release, forward-looking statements include, without limitation, those relating to the anticipated benefits to Vivos of the PDAC approval for the VidaSleep™ device as described herein. These statements involve significant known and unknown risks and are based upon several assumptions and estimates, which are inherently subject to significant uncertainties and contingencies, many of which are beyond Vivos’ control. Readers are cautioned that actual results may differ materially and adversely from those expressed or implied by such forward-looking statements. Factors that could cause actual results to differ materially include, but are not limited to: (i) the risk that Vivos may be unable to successfully implement sales, marketing and other strategies that increase revenues, (ii) the risk that some patients may not achieve the desired results from using Vivos’ products, (iii) the risk that published study data may not be predictive of results with Vivos treatment for all patients, (iv) risks associated with regulatory scrutiny of and adverse publicity in the sleep apnea diagnosis and treatment sector; (v) the risk that Vivos may be unable to secure additional financing when needed, if at all, or maintain its Nasdaq listing, (vi) market and other conditions that could impact Vivos’ business or ability to obtain financing (including whether insurance will cover the cost of Vivos treatment), and (vii) other risk factors described in Vivos’ filings with the Securities and Exchange Commission (“SEC”). Vivos’ filings can be obtained free of charge on the SEC’s website at www.sec.gov. Except to the extent required by law, Vivos expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Vivos’ expectations with respect thereto or any change in events, conditions, or circumstances on which any statement is based.
Media Inquiries:
Karla Jo Helms
JOTO PR™
727-777-4629
jotopr.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/ec018d95-bc0e-49fd-a261-23dddbef7a49








