Autonomix Medical, Inc. Reports Positive Outcomes in Initial Phase of First-in-Human Proof-of-Concept Trial in Pancreatic Cancer Pain and Initiates Market Expansion Study for Visceral Cancer Pain
Rhea-AI Summary
Autonomix Medical reports positive outcomes from its first-in-human proof-of-concept trial for pancreatic cancer pain treatment. The study enrolled 20 patients, with 19 receiving treatment through their innovative nerve ablation technology.
Key results include:
- 100% of femoral access patients responded to treatment
- 43.6% pain improvement at 7 days post-procedure
- 49.7% pain improvement at 4-6 weeks
- 100% of responding patients achieved zero opioid use at 7 days
- 73% remained opioid-free at 4-6 weeks
Based on these successful outcomes, Autonomix is expanding its study (PoC 2) to include additional visceral cancers and earlier-stage pancreatic cancer patients. The company's technology shows potential beyond cancer pain management, with possible applications in cardiology, hypertension, and chronic pain management. The expanded trial will begin in Q2 2025.
Positive
- 100% success rate in pain reduction for femoral access patients (16/16 patients)
- 53.3% pain reduction at 7 days and 59.2% at 4-6 weeks in responding patients
- 100% of responding patients achieved zero opioid use at 7 days
- 73% of responding patients maintained zero opioid use at 4-6 weeks
- No device or procedure-related serious adverse events reported
- Market expansion potential through PoC 2 study to double addressable market
- Technology platform can potentially address multiple indications beyond cancer pain
Negative
- 0% success rate in brachial access patients (0/3 patients)
- One patient (5%) could not be treated due to celiac trunk stenosis
- 6 patients died before 4-6 week follow-up due to disease progression
- Three patients' VAS scores missing at 4-6 weeks due to inability to travel
- 8 cases of arterial constrictions reported as adverse events
Insights
Autonomix's initial trial showed significant pain reduction in pancreatic cancer patients, eliminating opioid use with strong safety data.
Autonomix's first-in-human proof-of-concept study has delivered compelling efficacy signals in treating severe pancreatic cancer pain. The 20-patient trial demonstrated statistically significant pain reduction beginning at 24 hours and sustained through 4-6 weeks. The procedure reduced pain by
A critical procedural insight emerged: 100% of patients with femoral access (16 of 19) responded to treatment, while those with brachial access showed no improvement—providing valuable guidance for future protocol refinement.
The opioid reduction data is particularly noteworthy: every responding patient eliminated opioid use at 7 days, with
Safety data appears favorable, with no device or procedure-related serious adverse events. The technology's mechanism—using radiofrequency ablation with proprietary sensing technology—may enable more precise targeting of pain-signaling nerves.
Based on these results, Autonomix is expanding its clinical program to include additional visceral cancers and earlier-stage pancreatic cancer patients. This early data, while promising, will need confirmation in larger studies before potential regulatory clearance.
Autonomix's successful POC trial validates their platform technology and supports market expansion that could double their addressable opportunity.
Autonomix's positive clinical results represent a significant milestone validating their proprietary nerve ablation platform. The successful completion of their initial trial phase with clear efficacy signals establishes technical proof-of-concept while demonstrating meaningful clinical benefits in a difficult-to-treat population.
The strategic expansion into additional visceral cancers and earlier-stage pancreatic cancer is particularly noteworthy from a market opportunity perspective. By leveraging their existing technology platform across expanded indications, Autonomix could potentially double their addressable market—a classic platform expansion strategy employed by successful medical device companies.
The opioid reduction data represents a compelling value proposition in today's healthcare landscape. With heightened scrutiny around opioid prescribing and growing demand for effective alternatives, Autonomix's approach addresses a significant unmet need while potentially reducing healthcare costs associated with long-term opioid management.
The quality-of-life improvements reported—
Looking forward, management's commentary suggests broader applications beyond pain management, including potential uses in cardiology, hypertension, and chronic pain management—positioning the company with multiple potential revenue streams from their proprietary technology platform.
Initial trial phase (“PoC 1”) achieved key learnings and met all study objectives
Clinically meaningful pain reduction with
Company has initiated market expansion study (“PoC 2”) of additional visceral cancers, and earlier stage pancreatic cancer, to begin in Q2 2025
Additional indications potentially double the addressable market beyond pancreatic cancer pain
THE WOODLANDS, TX, April 30, 2025 (GLOBE NEWSWIRE) -- Autonomix Medical, Inc. (NASDAQ: AMIX) (“Autonomix” or the “Company”), a medical device company focused on advancing innovative technologies to revolutionize how diseases involving the nervous system are diagnosed and treated, today provided an update on the initial trial phase of its first-in-human proof-of-concept trial evaluating the safety and effectiveness of delivering transvascular energy to ablate relevant problematic nerves and mitigate pain in patients with severe pancreatic cancer pain.
The Company enrolled twenty (20) patients in PoC 1, including the first five (5) “lead-in” patients. Based on consistent, corresponding evidence that successfully met the trial objectives, the Company determined there was a sufficient number of patients and will conclude its initial phase of the study. Notable findings from the study include statistically significant pain relief as early as 24-hours post procedure, providing patients with rapid relief. Pain reduction remained consistently positive at 7-days and 4-6 weeks post-procedure. Patients also reported an improved quality of life during end-stage cancer while reducing their use of opioids, and the procedure demonstrated a strong safety profile. These promising results have led the Company to expand the protocol into a follow-on PoC 2 phase, now including pain management for additional visceral cancers as well as earlier stage pancreatic cancer patients experiencing moderate to severe pain.
Notable Topline Statistics and Key Learnings from the PoC 1 Phase (N=20)
- All patients entered the study with severe abdominal pain from unresectable pancreatic cancer and a life expectancy of 3 months or more.
- 19 of 20 enrolled patients were treated, and each assessed as successful catheter placement in the celiac trunk. One (1) patient was enrolled and not treated due to unsuccessful catheter placement because of an existing celiac trunk stenosis (narrowing of the vessel).
- There were no device or procedure-related serious adverse events. As to be expected with surgical procedures in seriously ill patient populations, there were 8 serious adverse events (6 subjects succumbed to their disease before the 4-6 week follow up, which were not related to the procedure, and 2 events resulting in hospitalization also unrelated to the procedure) and 14 adverse events (including 8 events of expected arterial constrictions due to spasms and temporary artery occlusion).
- 16 patients were treated using femoral access and three (3) patients using brachial access.
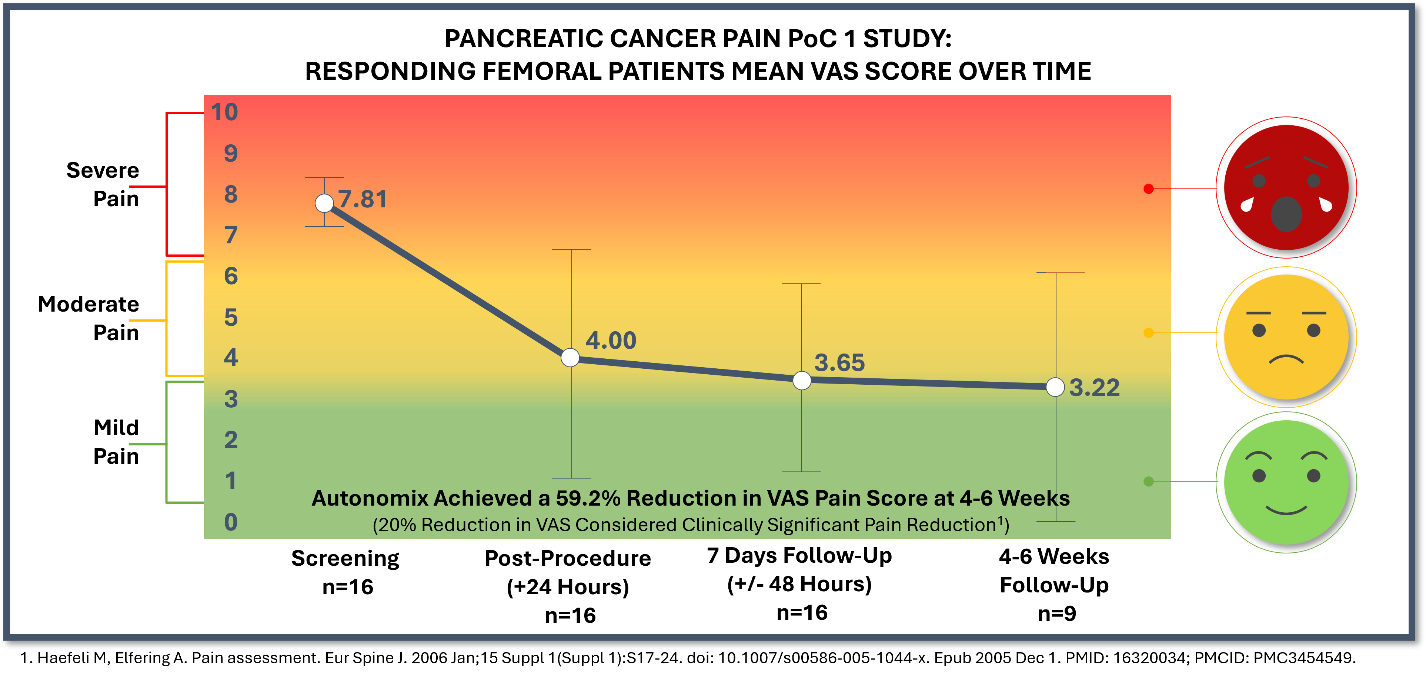
100% of patients (16) with femoral access responded to treatment, while the three (3) patients with brachial access showed no improvement in pain scores, representing a key procedural learning. - Across the total population (mITT – n=19), pain relief occurred as early as 24 hours post-procedure. At 7-days post-procedure, there was a mean pain reduction of 3.32 on the Visual Analog Scale (“VAS”) (baseline 7.61 to 4.29), or
43.6% improvement. At 4-6 weeks post-procedure, there was a mean pain reduction of 3.95 on the VAS pain scale (baseline of 7.95 to 4.00), or49.7% improvement. - Responding femoral patients (n=16) represented
84% of treated patients with a mean pain reduction of 4.16 on the VAS pain scale (baseline of 7.81 to 3.65), or53.3% improvement, at 7-days post-procedure. At 4-6 weeks post-procedure, there was a mean 4.67 reduction on the VAS pain scale (baseline of 7.89 to 3.22), or59.2% improvement. - At 7-days post-procedure, responding femoral patients reported a
76% improvement in global quality of health, a33% improvement in functional quality of life and a37% improvement in symptomatic quality of life. At 4-6 weeks post-procedure, responding femoral patients reported a42% improvement in global quality of health, a28% improvement in functional quality of life and a29% improvement in symptomatic quality of life. 100% of responding patients were able to go to zero opioid use at 7-days post-procedure, while73% of responding patients were at zero opioid use at 4-6 weeks post-procedure.- Three patient VAS scores were not reported at the 4-6 week post-procedure follow-up due to the inability to travel given the natural progression of their disease and will be recorded as missing data in the final report.
“We continue to be encouraged by the compelling results demonstrated in our PoC 1 phase, reinforced by consistent positive clinical outcomes observed at both the 7-day and 4-6 week post-procedure follow-ups. These findings provided a strong rationale for ceasing enrollment of this initial PoC 1 phase of the study and redirecting our clinical spending to additional targets that may be treated through the procedure. Most importantly, the significant reduction in pain and improvement in quality of life further validate our differentiated approach. Based on the data to date, we believe our technology has the potential to address a broader range of visceral cancers that signal pain through the Celiac Plexus, as well as earlier stage pancreatic cancers, in a follow-on PoC 2 phase of the trial. These results offer valuable insights that will guide future clinical studies, including our planned U.S. trials in 2025, bringing us closer to potential regulatory clearance,” Brad Hauser, CEO of Autonomix commented.
Based on the positive results demonstrated in the PoC 1 phase, Autonomix will initiate a follow-on PoC 2 phase in a market expansion opportunity that has the potential to double the addressable market beyond pancreatic cancer pain by evaluating additional visceral cancers that signal pain through the Celiac Plexus and earlier stage pancreatic cancers with moderate to severe pain. The current trial protocol will be amended to include the gathering of additional interventional pain management information that will continue to advance key learnings and be used to inform future clinical studies.
Autonomix’s technology constitutes a platform with the potential to address dozens of indications, including cardiology, hypertension and chronic pain management, across a wide disease spectrum. The PoC 2 phase will provide a concentrated focus on interventional cancer pain management applications like pancreatic, gall bladder, liver, and bile duct, with potential further expansion in oncology, gastroenterology, and other sectors where the Company has established key opinion leader relationships and emerging preclinical evidence.
Mr. Hauser continued, “The initial PoC 1 phase of the trial was a pivotal step in validating our approach that radiofrequency (“RF”) ablation can effectively block pain signals. With our proprietary sensing technology, we believe we can more precisely target overactive nerves, potentially improving response rates. Furthermore, by verifying that target nerves have been addressed after ablation, we aim to enhance the overall success rate of the procedure. Precision targeting is also central to our broader strategy of advancing beyond cancer pain in the celiac plexus to applications in cardiology, hypertension, and chronic pain management.”
For more information about the Company’s technology, please visit autonomix.com.
About the Proof-of-Concept Trial
The goal of the first-in-human proof-of-concept trial is to assess pain reduction via RF ablation. The Company’s catheter-based microchip sensing array used to detect and differentiate neural signaling was not used in this trial and will be evaluated in future studies.
The primary objective of the trial’s PoC 1 phase was to assess the success rate of ablating relevant nerves to mitigate pain in patients with pancreatic cancer pain utilizing RF ablation in a transvascular approach to the nerves in the region. Secondary objectives include assessing the incidence of device- and procedure-related adverse events up to 4-6 weeks post-procedure; estimating the change in pain levels from pre- to post-procedure; and estimating the change in quality of life from pre- to post-procedure. All patients who had a successful procedure were evaluated at 7 days, 4-6 weeks, and at 3 months post-procedure. All patients entered the study with severe abdominal pain from unresectable pancreatic cancer and had a life expectancy of 3 months or more.
About Autonomix Medical, Inc.
Autonomix is a medical device company focused on advancing innovative technologies to revolutionize how diseases involving the nervous system are diagnosed and treated. The Company’s first-in-class platform system technology includes a catheter-based microchip sensing array that may have the ability to detect and differentiate neural signals with greater sensitivity than currently available technologies. We believe this will enable, for the first time ever, transvascular diagnosis and treatment of diseases involving the peripheral nervous system virtually anywhere in the body.
We are initially developing this technology for the treatment of pain, with initial trials focused on pancreatic cancer, a condition that causes debilitating pain and is without a reliable solution. Our technology constitutes a platform to address dozens of potential indications, including cardiology, hypertension and chronic pain management, across a wide disease spectrum. Our technology is investigational and has not yet been cleared for marketing in the United States.
For more information, visit autonomix.com and connect with the Company on X, LinkedIn, Instagram and Facebook.
Forward Looking Statements
Some of the statements in this release are “forward-looking statements,” which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the timing of the commencement of the POC 2 phase, the potential of the technology to treat pain associated with pancreatic cancer, to successfully enroll patients within the specific timeframe, and to complete its clinical study in pancreatic cancer pain. Such forward-looking statements can be identified by the use of words such as “should,” “might,” “may,” “intends,” “anticipates,” “believes,” “estimates,” “projects,” “forecasts,” “expects,” “plans,” and “proposes.”
Although Autonomix believes that the expectations reflected in these forward-looking statements are based on reasonable assumptions, there are a number of risks and uncertainties that could cause actual results to differ materially from such forward-looking statements. You are urged to carefully review and consider any cautionary statements and other disclosures, including the statements made under the heading “Risk Factors” and elsewhere in the Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (“SEC”) on May 31, 2024 and in other filings made by us from time to time with the SEC. Forward-looking statements speak only as of the date of the document in which they are contained and Autonomix does not undertake any duty to update any forward-looking statements except as may be required by law.
Investor and Media Contact
JTC Team, LLC
Jenene Thomas
908.824.0775
autonomix@jtcir.com
Attachment









