Actinium Pharmaceuticals Presents New Preclinical Data Demonstrating Potent Anti-Tumor Activity of ATNM-400 Across Multiple Breast Cancer Subtypes Including Hormone Receptor-Positive, Triple-Negative, and Tamoxifen- and HER2 Therapy-Resistant Breast Cancer Models at SABCS 2025
Rhea-AI Summary
Actinium Pharmaceuticals (NYSE: ATNM) presented preclinical data for ATNM-400, an Actinium-225 antibody radioconjugate, at SABCS 2025 showing potent anti-tumor activity across HR+, TNBC, and tamoxifen- and trastuzumab-resistant breast cancer models.
Key findings include significant tumor-growth inhibition in HR+ and TNBC models, increased target expression and enhanced cytotoxicity in treatment-resistant models, combination-induced tumor regression in a trastuzumab-resistant model, sustained tumor uptake through 144 hours, and rapid clearance from normal organs suggesting a differentiated tolerability profile.
Positive
- Potent tumor-growth inhibition in HR+ and TNBC models
- Enhanced cytotoxicity in tamoxifen- and trastuzumab-resistant models
- Combination with SOC agents produced in vivo tumor regression
- Sustained tumor uptake through 144 hours with rapid normal-organ clearance
- Pan-tumor applicability supported by prostate and lung cancer data
Negative
- Data are preclinical; no clinical efficacy or safety in humans yet
- No quantitative human safety or tolerability data provided
- Potential risks of translation to clinic not addressed in preclinical poster
News Market Reaction
On the day this news was published, ATNM gained 4.14%, reflecting a moderate positive market reaction. This price movement added approximately $2M to the company's valuation, bringing the market cap to $45M at that time.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
ATNM was up 2.11% while close peers were mixed: notable decliners like VTVT -5.15% and GNTA -4.62%, with PDSB modestly higher at +1.0%, suggesting a stock-specific move around ATNM-400 data.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Dec 01 | Breast data preview | Positive | -0.7% | Previewed potent ATNM-400 efficacy in TNBC and resistant breast cancer models. |
| Nov 17 | Strategy/market focus | Positive | +14.2% | Outlined ATNM-400 pan-tumor strategy and large targeted radiotherapy market context. |
| Nov 04 | Breast data announcement | Positive | -2.2% | Announced upcoming SABCS breast data and reinforced multi-indication ATNM-400 profile. |
| Nov 03 | Conference participation | Neutral | -3.5% | Notified participation in Stephens biotech virtual fireside chat and investor meetings. |
| Oct 27 | Lung cancer data | Positive | -8.4% | Reported superior ATNM-400 NSCLC tumor inhibition and complete regressions with osimertinib. |
Recent ATNM-400 data releases have often seen weak or negative next-day reactions despite positive preclinical signals, with only one clearly positive price response among multiple upbeat updates.
Over the last few months, Actinium has repeatedly highlighted ATNM-400 as a pan-tumor Ac‑225 radioconjugate across breast, prostate and lung cancer. Releases on Oct 27, Nov 4, and Dec 1, 2025 all reported strong preclinical efficacy, including in resistant breast cancer models, yet price reactions were often negative. A Nov 17 feature on the broader radiotherapy opportunity and IP estate drew the strongest positive move. Today’s SABCS 2025 preclinical breast cancer data extend this ATNM‑400 narrative within the same strategic framework.
Market Pulse Summary
This announcement adds detailed preclinical evidence that ATNM-400, an Actinium‑225 antibody radioconjugate, produced robust anti‑tumor activity and favorable tolerability across hormone receptor–positive, triple‑negative, and therapy‑resistant breast cancer models. It reinforces earlier communications on ATNM‑400’s pan‑tumor potential in prostate and lung cancer. Within the broader trajectory, investors may watch for progression from preclinical to clinical studies, evolving safety and efficacy data, and how these results intersect with Actinium’s ongoing net loss profile and cash usage.
Key Terms
actinium-225 medical
antibody radioconjugate medical
triple-negative breast cancer medical
non-small cell lung cancer medical
double-strand dna breaks medical
interstitial lung disease medical
alpha-particle medical
xenograft models medical
AI-generated analysis. Not financial advice.
- ATNM-400's target antigen is overexpressed in breast cancer and its expression is further increased in breast cancer cells resistant to standard of care endocrine therapy, tamoxifen and the HER2 therapy trastuzumab
- The Actinium-225 alpha-emitter payload of ATNM-400 induced irreversible double-strand DNA breaks and has the potential to produce potent localized tumor killing with reduced off-target lung toxicity that limits the use of antibody drug conjugates
- Data supports the continued development of ATNM-400 as a monotherapy or combination therapy to address multiple breast cancer subtypes with limited treatment options
Highlights from the SABCS 2025 Poster Presentation
The poster, titled "Anti-Tumor Activity of ATNM-400, a First-in-Class Actinium-225 Antibody Radioconjugate, in Hormone-Positive, Triple-Negative, Tamoxifen-Resistant and Trastuzumab-Resistant Breast Cancer Models," showcases the following key findings:
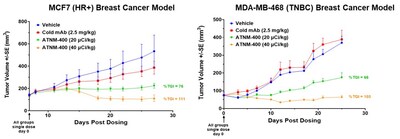
Potent Efficacy Across Breast Cancer Subtypes: ATNM-400 demonstrated significant tumor-growth inhibition (TGI) in HR+ (MCF7) and TNBC (MDA-MB-468) in vivo models, with all treatment regimens well tolerated and no significant changes in body weight observed.
Potent Activity in Standard-of-care (SOC) Treatment-Resistant Breast Cancer Models:
- Trastuzumab-resistant BT474-Clone5 breast cancer cells or Tamoxifen-resistant MCF7-Tam1 breast cancer cells exhibited increased target expression, resulting in enhanced in vitro cytotoxicity with ATNM-400.
- Combining ATNM-400 with either trastuzumab or tamoxifen resulted in greater cytotoxicity versus monotherapy and produced in vivo tumor regression in the trastuzumab-resistant model.
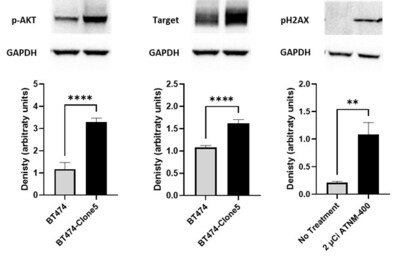
Mechanistic Evidence of Irreversible DNA Damage
- Activation of phosphorylation of AKT (pAKT) was observed in trastuzumab resistant BT474-Clone5 breast cancer cells, as well as a significant increase in the total level of the ATNM-400 target antigen in the in vivo trastuzumab-resistant breast cancer model.
- ATNM-400 treatment of these trastuzumab-resistant breast cancer cells caused significant increase in phosphorylated H2AZ (pH2AX), consistent with alpha-particle–driven double-strand DNA damage.
Favorable Biodistribution: Sustained tumor uptake in a breast cancer model through 144 hours and rapid clearance from normal organs supports a potentially differentiated safety profile.
Pan-Tumor Potential: These results, together with previously published ATNM-400 data in prostate and lung cancer, reinforce the program's broad applicability across solid tumors.
Dr. Aditya Bardia, Professor of Medicine and Program Director, Breast Medical Oncology at the Geffen School of Medicine, University of California Los Angeles, was an author on the ATNM-400 SABCS poster.
"Patients whose tumors progress after endocrine therapy or HER2-targeted therapies face limited and often toxic treatment options. The data presented at SABCS show that ATNM-400 generates potent, targeted DNA damage even in highly resistant breast cancer models, while maintaining a favorable tolerability profile. This therapeutic approach has the potential to address some of the most pressing unmet needs in breast cancer care, particularly for those who have exhausted established modalities", said Dr. Bardia.
Breast cancer remains a heterogeneous disease with significant unmet need, particularly for patients who relapse following endocrine or HER2-directed therapies—an issue impacting 20–
Sandesh Seth, Chairman and CEO of Actinium Pharmaceuticals, added, "ATNM-400 represents an important component of Actinium's strategy to develop next-generation radiotherapeutics that can meaningfully expand treatment options for patients with hard-to-treat solid tumors. The breadth of activity seen in both endocrine-resistant and HER2-resistant breast cancer reinforces ATNM-400's potential to become a differentiated, first-in-class therapy. Beyond breast cancer, we have presented compelling ATNM-400 data in prostate cancer and lung cancer that demonstrate its pan-tumor potential. These data further support our excitement for ATNM-400 and our plans to accelerate development of ATNM-400 as we advance toward clinical readiness."
The ATNM-400 SABCS poster presentation can be access on Actinium's investor relations website HERE.
About ATNM-400
ATNM-400 is a highly innovative, first-in-class, and multi-indication Actinium-225 (Ac-225) targeted radiotherapy candidate in development for prostate cancer, non-small cell lung cancer (NSCLC) and breast cancer. ATNM-400 is highly differentiated in prostate cancer as it targets a distinct non-PSMA protein strongly implicated in prostate cancer disease biology including progression and treatment resistance. Unlike 177Lu-PSMA-617, the active agent in Pluvicto® and the majority of radiotherapies under development, which rely on PSMA targeting, ATNM-400 is designed to maintain efficacy in low-PSMA or high-PSMA resistant disease, a major unmet clinical need as up to
Prostate cancer is the most commonly diagnosed cancer in men, with ~1.5 million new cases globally and over 313,000 expected in the
About Actinium Pharmaceuticals, Inc.
Actinium is a pioneer in the development of targeted radiotherapies intended to meaningfully improve patient outcomes. ATNM-400, Actinium's lead product candidate, is a novel, first-in-class, and multi-indication Actinium-225 (Ac-225) in development for prostate cancer, non-small cell lung cancer (NSCLC) and breast cancer. The antigen specifically targeted by ATNM-400 is highly expressed in metastatic castration-resistant prostate cancer (mCRPC), contributes directly to disease progression, poorer survival outcomes, and continues to be expressed at a high level even after androgen receptor inhibitor (ARPI) and Pluvicto® treatment. ATNM-400 is supported by preclinical data demonstrating tumor-specific uptake, higher efficacy than androgen receptor inhibitor enzalutamide (Xtandi®) and 177Lu-PSMA-617 radiotherapy, the active agent in Pluvicto®, durable tumor control and potent efficacy in prostate cancer models resistant to both enzalutamide and 177Lu-PSMA-617. In addition, ATNM-400 has demonstrated synergy with enzalutamide. In NSCLC, ATNM-400 showed superior efficacy to EGFR targeting therapies including osimertinib (TARGRISSO®, AstraZeneca), Dato-DXd (DATROWAY®, AstraZeneca/Daiichi Sankyo) and amivantamab (RYBREVANT®, J&J) with synergistic activity in combination with osimertinib. In breast cancer, ATNM-400 showed potent anti-tumor activity across multiple breast cancer subtypes including hormone receptor-positive, triple-negative, and tamoxifen- and HER2 therapy-resistant breast cancer models. The data generated to date with ATNM-400 supports its potential across treatment settings to be used either as a monotherapy, or in combination or sequenced with other therapies. Actinium's most advanced product candidate in development is Actimab-A, a CD33 targeting therapeutic, that is a potential backbone therapy for acute myeloid leukemia (AML) and other myeloid malignancies leveraging the mutation agnostic alpha-emitter radioisotope payload Actinium-225 (Ac-225). Actimab-A has demonstrated potential activity in relapsed and refractory acute myeloid leukemia (r/r AML) patients in combination with the chemotherapy CLAG-M including high rates of Complete Remissions (CR) and measurable residual disease (MRD) negativity leading to improved survival outcomes and is being advanced to a pivotal Phase 2/3 trial. In addition, Actinium is engaged with the National Cancer Institute (NCI) under a Cooperative Research and Development Agreement (CRADA) for development of Actimab-A in AML and other myeloid malignancies. The first clinical trial under the CRADA will evaluate the triplet combination comprised of Actimab-A, Venetoclax (Abbvie/Roche) an oral Bcl-2 inhibitor and ASTX-727 (Taiho Oncology, an Otsuka holdings company) a novel oral hypomethylating agent (HMA) in frontline acute myeloid leukemia (AML) patients. Additionally, Actinium is developing Actimab-A as a potential pan tumor therapy in combination with PD-1 checkpoint inhibitors including KEYTRUDA® and OPDIVO® by depleting myeloid derived suppressor cells (MDSCs), which represents a potential multi-billion-dollar addressable market. Iomab-ACT, Actinium's next generation conditioning candidate, is being developed with the goal of improving patient access and outcomes for potentially curative cell and gene therapies. Iomab-B is an induction and conditioning agent prior to bone marrow transplant in patients with r/r AML, which Actinium is seeking a potential strategic partner for the
For more information, please visit: https://www.actiniumpharma.com/
Forward-Looking Statements
This press release may contain projections or other "forward-looking statements" within the meaning of the "safe-harbor" provisions of the private securities litigation reform act of 1995 regarding future events or the future financial performance of the Company which the Company undertakes no obligation to update. These statements are based on management's current expectations and are subject to risks and uncertainties that may cause actual results to differ materially from the anticipated or estimated future results, including the risks and uncertainties associated with preliminary study results varying from final results, estimates of potential markets for drugs under development, clinical trials, actions by the FDA and other governmental agencies, regulatory clearances, responses to regulatory matters, the market demand for and acceptance of Actinium's products and services, performance of clinical research organizations and other risks detailed from time to time in Actinium's filings with the Securities and Exchange Commission (the "SEC"), including without limitation its most recent annual report on form 10-K, subsequent quarterly reports on Forms 10-Q and Forms 8-K, each as amended and supplemented from time to time.
Investors:
investorrelations@actiniumpharma.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/actinium-pharmaceuticals-presents-new-preclinical-data-demonstrating-potent-anti-tumor-activity-of-atnm-400-across-multiple-breast-cancer-subtypes-including-hormone-receptor-positive-triple-negative-and-tamoxifen--and-her2-thera-302640194.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/actinium-pharmaceuticals-presents-new-preclinical-data-demonstrating-potent-anti-tumor-activity-of-atnm-400-across-multiple-breast-cancer-subtypes-including-hormone-receptor-positive-triple-negative-and-tamoxifen--and-her2-thera-302640194.html
SOURCE Actinium Pharmaceuticals, Inc.