Immatics Achieves Clinical Proof-of-Concept of its Next-Generation TCR Bispecific (TCER®) Pipeline with Data on IMA402 (PRAME) and IMA401 (MAGEA4/8) and Announces Next Development Steps
Rhea-AI Summary
Immatics (NASDAQ: IMTX) reported Phase 1a dose‑escalation data for its next‑generation TCR Bispecifics, IMA402 (PRAME) and IMA401 (MAGEA4/8), and announced Phase 1b plans on November 12, 2025. IMA402 at the RP2D range (10–30 mg) produced a 30% cORR (6/20) across indications, including 29% in melanoma (4/14), with deep durable responses (two complete metabolic responses ongoing 8 and 18 months). Median PFS was 4.8 months (mFU 6.8); 1‑year OS rate was 94%. IMA401 at ≥1 mg showed activity: 25% cORR in head and neck and 29% in melanoma, with a longest response >2 years. Both candidates showed favorable tolerability; provisional RP2D ranges were identified (IMA402: 10–30 mg; IMA401: 1–2 mg).
Positive
- IMA402 cORR 30% (6/20) at RP2D
- IMA402 melanoma cORR 29% (4/14) with two complete metabolic responses
- Median PFS 4.8 months for IMA402 at mFU 6.8 months
- IMA401 cORR 25% in head and neck cancer (2/8) at ≥1 mg
- Provisional RP2D ranges set for both programs (IMA402 10–30 mg; IMA401 1–2 mg)
Negative
- Small efficacy cohorts: IMA402 RP2D efficacy‑evaluable n=20
- Three DLTs at 2.5 mg observed for IMA401 during escalation
News Market Reaction – IMTX
On the day this news was published, IMTX gained 16.10%, reflecting a significant positive market reaction. Argus tracked a peak move of +44.5% during that session. Our momentum scanner triggered 39 alerts that day, indicating elevated trading interest and price volatility. This price movement added approximately $183M to the company's valuation, bringing the market cap to $1.32B at that time.
Data tracked by StockTitan Argus on the day of publication.
Company to host conference call and webcast today, November 12, at 8:30 am EST/2:30 pm CET
- IMA402 and IMA401 TCR Bispecifics showed favorable tolerability at RP2D as well as deep and durable responses in heavily pre-treated, last-line patients with a range of solid tumors
- IMA402 PRAME Bispecific at RP2D range resulted in a
30% cORR (6/20) across all indications, including29% (4/14) in melanoma and 2/3 confirmed responses in ovarian carcinoma - IMA401 MAGEA4/8 Bispecific at ≥1 mg resulted in a
25% cORR (2/8) in head and neck cancer,29% cORR (2/7) in melanoma and promising clinical activity in sqNSCLC - Phase 1a dose escalation completed for both trials; data support IMA402 PRAME Bispecific development opportunities in cutaneous melanoma, gynecologic cancers and in combination with IMA401 MAGEA4/8 Bispecific in sqNSCLC
- Phase 1b dose expansion for IMA402 initiated
- Conference call and webcast can be accessed here
Houston, Texas and Tuebingen, Germany, November 12, 2025 – Immatics N.V. (NASDAQ: IMTX, “Immatics” or the “Company”), a clinical-stage biopharmaceutical company and the global leader in precision targeting of PRAME, today announced updated Phase 1a dose escalation data from both product candidates in its TCR Bispecifics (TCER®) pipeline, IMA402 PRAME Bispecific and IMA401 MAGEA4/8 Bispecific, as well as next steps for clinical development.
“Our off-the-shelf TCR Bispecifics have a proprietary next-generation format with half-life extension that is designed to combine optimized tolerability and potent anti-tumor activity while supporting patient-convenient dosing,” said Carsten Reinhardt, M.D., Ph.D., Chief Development Officer at Immatics. “We have now achieved clinical proof-of-concept for both product candidates and seen their potential to make a meaningful impact on patients with limited treatment options through deep and durable responses. We look forward to continuing to drive the development of our bispecifics to advance accessible, innovative therapies that can reach more patients and make a lasting difference in cancer care.”
“Today marks the beginning of a new phase for Immatics, expanding our reach beyond cell therapy and establishing a leading position in the TCR Bispecifics field, with a clear commitment to advancing the clinical development of our bispecifics pipeline,” said Harpreet Singh, Ph.D., CEO and Co-Founder of Immatics. “Building on these data, we are excited to evaluate our IMA402 PRAME Bispecific now across multiple targeted cancer patient populations with significant unmet treatment needs and in potentially synergistic combinations. We are especially enthusiastic about the potential for profound benefit by combining IMA402 with IMA401, our MAGEA4/8 Bispecific, in patients with squamous non-small cell lung cancer, a large, highly underserved and difficult-to-treat indication.”
Carsten Reinhardt, M.D., Ph.D., and Harpreet Singh, Ph.D., will present the complete TCR Bispecifics dataset and next development steps during a conference call and webcast today, November 12, at 8:30 am EST/2:30 pm CET. The presentation is accessible on the ‘Events & Presentations’ page on the Investors & Media section of the Company’s website.
IMA402 PRAME Bispecific Phase 1a Dose Escalation Data Summary
Patient Population: Advanced metastatic solid tumors with no available treatment options
As of the data cutoff on September 26, 2025, 80 heavily pre-treated patients (median of three prior systemic treatments) with recurrent and/or refractory solid tumors1 were treated with escalating dose levels of IMA402 monotherapy ranging from 0.02 mg to 30 mg. The safety population includes all 80 patients treated with IMA402. 29 patients received doses in the recommended Phase 2 dose (RP2D range) (10 to 30 mg) and, thereof, 20 patients were efficacy-evaluable2, including 14 patients with melanoma (12 cutaneous, 1 uveal, 1 unknown primary), 3 patients with ovarian carcinoma and 3 patients with other solid cancers3.
Safety: Treatment with IMA402 showed favorable tolerability
IMA402 showed favorable tolerability across a wide dose range in the 80 patients treated. The most frequent treatment-related adverse events (AEs) were expected and transient lymphopenia, consistent with the mechanism of action, and low-grade cytokine release syndrome (CRS): Grade 1:
Phase 1a dose escalation in the monotherapy setting has been completed. The maximum tolerated dose (MTD) has not been reached. The provisional RP2D range has been identified at 10 to 30 mg. The Phase 1b dose expansion is ongoing at two distinct doses within the RP2D range, and the evaluation of IMA402 in combination with an immune checkpoint inhibitor has been initiated.
Anti-tumor Activity and Durability: Deep and durable responses observed at RP2D range
IMA402 showed a clear dose-response relationship across three different dose groups.
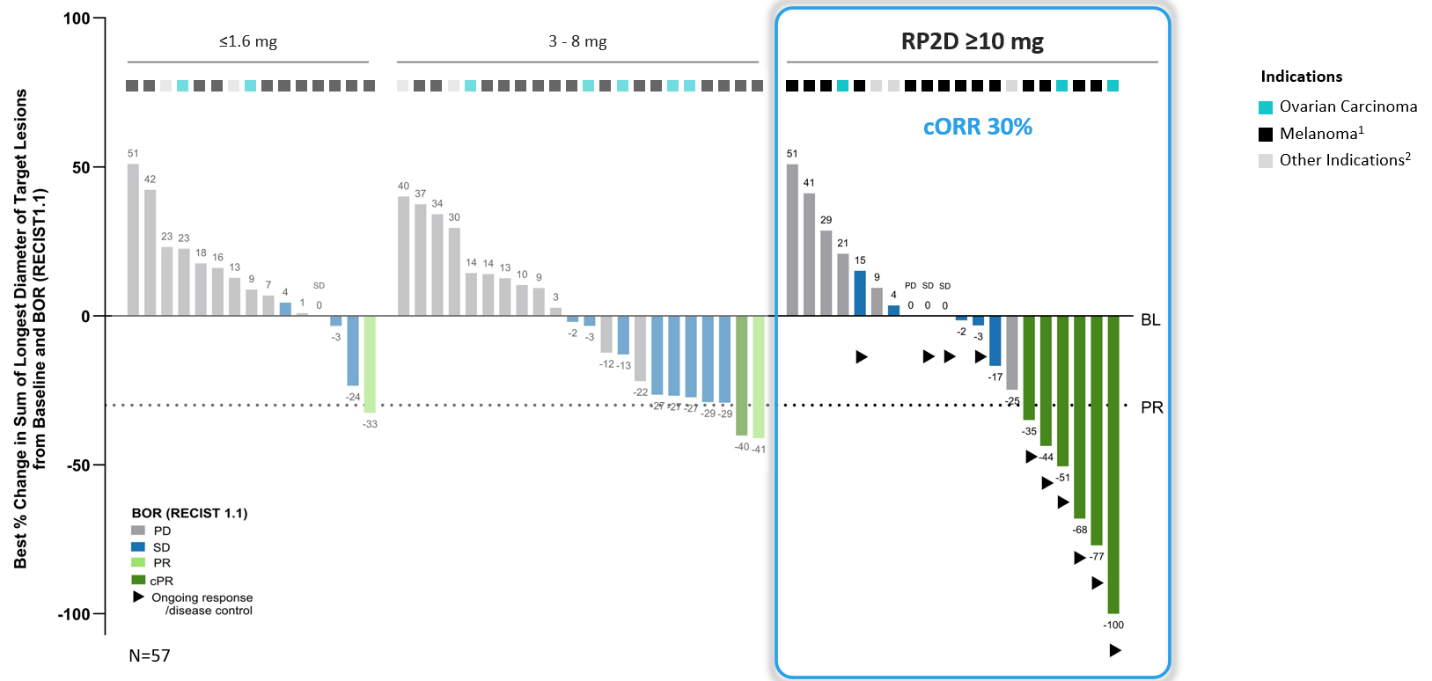
1 Melanoma includes cutaneous melanoma, melanoma of unknown primary, uveal melanoma; 2 Other indications include endometrioid carcinoma, synovial sarcoma and one patient with sqNSCLC at 1.6 mg; BL: baseline; BOR: best overall response; cORR: confirmed objective response rate; cPR: confirmed partial response; PD: progressive disease; PR: partial response; SD: stable disease; RECIST: response evaluation criteria in solid tumors; RP2D: recommended phase 2 dose
Several patients dosed with IMA402 at the RP2D range were observed to have deep and durable responses. All 6 confirmed objective responses were ongoing as of data cutoff, including two complete metabolic responses in cutaneous and uveal melanoma, ongoing at 8 and 18 months, respectively, as well as one confirmed partial response in ovarian carcinoma with -
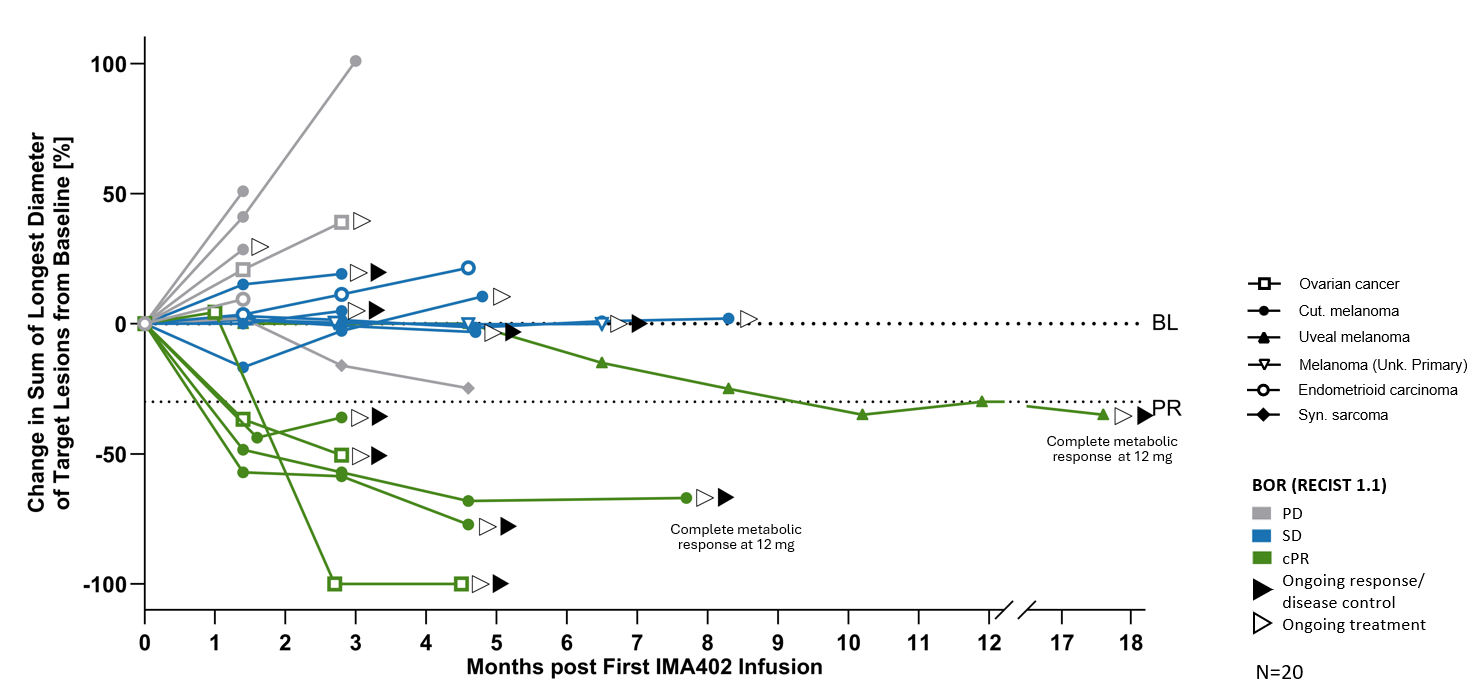
BL: baseline; cPR: confirmed partial response; PD: progressive disease; PR: partial response; SD: stable disease
Deep and durable responses at RP2D range (RECIST 1.1)
| All Indications | Melanoma | Ovarian Carcinoma | |
| cORR | 2/3 | ||
| mDOR, mFU month | Not reached 4.2 | Not reached 7.3 | Not reached 2.2 |
| Tumor shrinkage | 2/3 | ||
| DCR (at week 6) | 2/3 |
mDOR: median duration of response; mFU: median follow-up; DCR: disease control rate
For patients across all indications treated within the RP2D range early, promising progression-free survival (PFS) and overall survival (OS) were observed:
- Median PFS was 4.8 months at a mFU of 6.8 months; 6-month PFS rate was
45% - Median iPFS4 was not reached at a mFU of 6.3 months; 6-month iPFS rate was
58% - Median OS was not reached at a mFU of 5.4 months; 1-year OS rate was
94%
Clinical Development Opportunities for IMA402 PRAME Bispecific
Based on the promising Phase 1a dose escalation data, Immatics is advancing its IMA402 PRAME Bispecific into Phase 1b dose expansion at two distinct doses to determine the final RP2D, both as a monotherapy and in combination with an immune checkpoint inhibitor with a focus on melanoma and gynecologic cancers in 2026. Depending on the outcomes of these Phase 1b cohorts, the Company would seek to convert existing Phase 1b cohorts into Phase 2 trials, which will then have the potential to become registration-directed. As part of its strategy to maximize the IMA402 opportunity, the Company is also exploring the option to initiate additional Phase 1b cohorts in 2026 to determine the monotherapy and combination potential of IMA402 with immune checkpoint inhibitors and standard of care in late as well as earlier treatment lines. As an additional opportunity, the Company is exploring the potential combination of IMA402 with IMA401 MAGEA4/8 in squamous non-small cell lung cancer (sqNSCLC) and potentially other solid tumor indications.
IMA401 MAGEA4/8 Bispecific Phase 1a Data Summary
Patient Population: Heavily pre-treated patients with a broad range of tumor types with no available treatment options
As of the data cutoff on September 26, 2025, 55 heavily pretreated patients (median of four prior systemic treatments) with recurrent and/or refractory solid tumors5 were treated with escalating dose levels of IMA401 ranging from 0.0066 mg to 2.5 mg with or without an immune checkpoint inhibitor (ICI, pembrolizumab). The safety population includes all 55 patients treated with IMA401 as a monotherapy (n=46) or in combination with pembrolizumab (n=9). 44 patients were treated with doses from 1 to 2.5 mg, and thereof 38 were evaluable for efficacy6. All efficacy-evaluable patients treated with IMA401 in combination with pembrolizumab (n=4) had progressed on prior immune checkpoint inhibitor treatments.
Safety: Treatment with IMA401 showed favorable tolerability at RP2D
The most frequent and relevant treatment-related adverse events (AEs) across all 55 patients treated with IMA401 were low-grade cytokine release syndrome (CRS) (
The maximum tolerated dose (MTD) has not been reached; three dose-limiting events were observed at 2.5 mg. The Phase 1a dose escalation has been completed, and the provisional RP2D range has been identified at 1-2 mg. At RP2D, the tolerability profile was favorable.
Anti-tumor Activity and Durability: Promising clinical activity and deep and durable responses were observed in patients with head and neck cancer, melanoma and lung cancer treated at ≥1 mg
Patients in three focus indications treated with ≥1 mg of IMA401 as a monotherapy or in combination with pembrolizumab demonstrated clinical activity:
- Head and neck cancer: cORR of
25% (2/8), disease control rate of63% (5/8) - Melanoma: cORR of
29% (2/7), disease control rate of57% (4/7) - Squamous non-small-cell lung cancer: 1 partial response at first scan for a heavily pre-treated, ICI-resistant patient, 1 patient with stable disease for >4 months and overall survival of approximately 16 months, 1 patient with progressive disease with shrinkage of liver target lesions
The duration of all confirmed responses was longer than 6 months post treatment, with the longest response ongoing over 2 years in a patient with advanced cutaneous melanoma.
Clinical Development Opportunity for IMA401 MAGEA4/8 Bispecific
Consistent with Immatics’ focus on advancing its PRAME franchise, the Company is exploring IMA401 in combination with IMA402, starting with squamous non-small cell lung cancer (sqNSCLC). Based on the clinical proof-of-concept of both bispecific candidates, including the initial promising activity of IMA401 in head and neck cancer and sqNSCLC, as well as preclinical proof-of-concept data, Immatics is well-positioned to assess the synergistic potential of combining two different bispecifics, IMA402 targeting PRAME and IMA401 targeting MAGEA4/8, with and without a checkpoint inhibitor. As over
About Immatics TCR Bispecifics (TCER®)
Immatics’ next-generation half-life extended TCER® molecules are antibody-like “off-the-shelf” biologics that leverage the body’s immune system by redirecting and activating T cells towards cancer cells expressing a specific tumor target. The design of the TCER® molecules enables the activation of any T cell in the body to attack the tumor, regardless of the T cells’ intrinsic specificity. Immatics’ proprietary biologics are engineered with two binding regions: a TCR domain and a T cell recruiter domain. The TCER® format is designed to maximize efficacy while minimizing toxicities in patients. It contains a high-affinity TCR domain that is designed to bind specifically to the cancer target peptide on the cell surface presented by an HLA molecule. The antibody-derived, low-affinity T cell recruiter domain is directed against the TCR/CD3 complex and recruits a patient’s T cells to the tumor to attack the cancer cells. With a low-affinity recruiter aiming for optimized biodistribution and enrichment of the molecule at the tumor site instead of the periphery, TCER® are engineered to reduce the occurrence of immune-related adverse events, such as cytokine release syndrome. In addition, the TCER® format consists of an Fc-part conferring half-life extension, stability, and manufacturability. TCER® are “off-the-shelf” biologics and thus immediately available for patient treatment. They can be distributed through standard pharmaceutical supply chains and provide the opportunity to reach a large patient population without the need for specialized medical centers.
About PRAME
PRAME is a target expressed in more than 50 cancers. Immatics is the global leader in precision targeting of PRAME and has the broadest PRAME franchise with the most PRAME indications and modalities. The Immatics PRAME franchise currently includes three product candidates, two therapeutic modalities and a combination therapy that target PRAME: anzu-cel (IMA203) PRAME cell therapy, IMA203CD8 PRAME cell therapy (GEN2), IMA402 PRAME bispecific, anzu-cel in combination with Moderna’s PRAME cell therapy enhancer.
About IMA402 PRAME Bispecific
IMA402 is a molecule from Immatics’ TCR Bispecifics (TCER®) pipeline directed against an HLA-A*02:01-presented peptide derived from PRAME
IMA402 is currently being evaluated in a Phase 1 trial in patients with solid tumors expressing PRAME. IMA402 is part of Immatics’ strategy to leverage the full clinical potential of targeting PRAME, one of the most promising targets for TCR-based therapies.
About IMA401 MAGEA4/8 Bispecific
IMA401 is a molecule from Immatics’ TCR Bispecifics pipeline that targets an HLA-A*02:01-presented peptide derived from two different cancer-associated proteins, melanoma-associated antigen 4 and/or 8 (“MAGEA4/8”). The MAGEA4/8 peptide has been identified and validated by Immatics’ proprietary mass spectrometry-based target discovery platform XPRESIDENT® and is presented at a 5-fold higher target density (copy number per tumor cell) than the MAGEA4 peptide targeted in other clinical trials.
IMA401 is currently being evaluated in a Phase 1 basket trial in patients with MAGEA4/8-positive solid tumors. The MAGEA4/8 peptide has a high prevalence in several solid tumor indications such as head and neck squamous cell carcinoma (HNSCC), squamous cell non-small cell lung cancer (sqNSCLC), as well as melanoma and other solid cancer types.
About Immatics
Immatics is committed to making a meaningful impact on the lives of patients with cancer. We are the global leader in precision targeting of PRAME, a target expressed in more than 50 cancers. Our cutting-edge science and robust clinical pipeline form the broadest PRAME franchise with the most PRAME indications and modalities, spanning TCR T-cell therapies and TCR bispecifics.
Immatics intends to use its website www.immatics.com as a means of disclosing material non-public information. For regular updates, you can also follow us on LinkedIn and Instagram.
Forward-Looking Statements
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or the Company’s future financial or operating performance. For example, statements concerning timing of data read-outs for product candidates, observations from the Company’s clinical trials, the timing, outcome and design of clinical trials, the nature of clinical trials (including whether such clinical trials will be registration-enabling), the timing of IND, CTA or BLA filings, estimated market opportunities of product candidates, the Company’s focus on partnerships to advance its strategy, and other metrics are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “plan”, “target”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management's control including general economic conditions and other risks, uncertainties and factors set forth in the Company’s Annual Report on Form 20-F and other filings with the Securities and Exchange Commission (SEC). Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. The Company undertakes no duty to update these forward-looking statements. All the scientific and clinical data presented within this press release are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification.
For more information, please contact:
Media
Trophic Communications
Phone: +49 151 74416179
immatics@trophic.eu
Immatics N.V.
Jordan Silverstein
Head of Strategy
Phone: +1 346 319-3325
InvestorRelations@immatics.com
- END -
1 Cutaneous melanoma, uveal melanoma, synovial sarcoma, endometrial carcinoma, ovarian cancer, squamous non-small cell lung cancer.
2 Efficacy-evaluable patients: All patients treated as of June 26, 2025 (who had the opportunity for at least 3 months follow-up or who discontinued early due to disease progression or death), tested positive or not tested/not evaluable for PRAME and received ≥4 infusions as defined per protocol (thereof 3 step doses, currently at 0.03 mg/0.3 mg/6 mg, and 1 target dose).
3 N=2 endometrioid carcinoma, n=1 synovial sarcoma.
4 iRECIST, developed by the RECIST Working Group, adapts the RECIST 1.1 definition for progression of immunotherapies by introducing unconfirmed (iUPD) and confirmed (iCPD) progression to account for atypical response patterns. Patients with iUPD not confirmed at a subsequent scan but turning into SD or response are not considered progressive according to iRECIST. PFS (according to RECIST 1.1) and iPFS (according to iRECIST) are prospectively defined co-secondary endpoints in the IMA402 trial protocol to provide a balanced view of efficacy.
5 Basket trial with >15 different tumor indications.
6 Efficacy-evaluable patients: All patients treated as of June 26, 2025 (who had the opportunity for at least 3 months follow-up or who discontinued early due to disease progression or death), and received ≥4 infusions as defined per protocol (thereof 3 step doses, currently at 0.3 mg/0.6 mg/1 mg, and 1 target doses).
Attachment








