Immatics Highlights Compelling Anti-Tumor Activity of Anzu-cel PRAME Cell Therapy in Metastatic Uveal Melanoma at the ESMO 2025 Presidential Symposium
Rhea-AI Summary
Immatics (NASDAQ: IMTX) reported updated Phase 1b data for one-time anzu-cel (anzutresgene autoleucel, IMA203) PRAME cell therapy in 16 metastatic uveal melanoma patients presented at ESMO 2025 on October 20, 2025.
Key outcomes: cORR 67% (10/15), mDOR 11.0 months, mPFS 8.5 months (median follow-up 10.4 months) and mOS not reached at 14.3 months mFU; median liver target lesion shrinkage -49.6%. Safety showed mostly Grade 1–2 CRS; Grade 3 CRS 18.8% and no anzu-cel–related Grade 5 events. A Phase 2 cohort (~30 patients) in metastatic uveal melanoma has been initiated.
Positive
- Confirmed objective response rate 67% (10/15)
- Median duration of response 11.0 months
- Median PFS 8.5 months with 69% six-month PFS
- Median liver lesion shrinkage 49.6%
- Phase 2 cohort initiated with ~30 planned patients
Negative
- Small dataset of only 16 treated patients
- Median follow-up limited to 14.3 months for OS
- Grade 3 cytokine release syndrome in 18.8% of patients
- Frequent lymphodepletion-related cytopenias as TEAs
News Market Reaction
On the day this news was published, IMTX gained 11.04%, reflecting a significant positive market reaction. Our momentum scanner triggered 4 alerts that day, indicating moderate trading interest and price volatility. This price movement added approximately $131M to the company's valuation, bringing the market cap to $1.32B at that time.
Data tracked by StockTitan Argus on the day of publication.
- One-time infusion of anzu-cel (anzutresgene autoleucel, IMA203) PRAME cell therapy in 16 patients with metastatic uveal melanoma in the ongoing Phase 1b trial continues to show strong clinical benefit with more mature data: cORR of
67% , mDOR of 11.0 months, mPFS of 8.5 months and mOS not reached at 14.3 months mFU - Anzu-cel maintains a favorable tolerability profile in metastatic uveal melanoma
- Anti-tumor activity observed across metastases throughout the body, including liver lesions, and in patients who received prior treatment with TCR-based therapies
- Given the promising clinical activity of anzu-cel and high PRAME prevalence in uveal melanoma, a Phase 2 cohort has been initiated in patients with metastatic uveal melanoma
Houston, Texas and Tuebingen, Germany, October 20, 2025 – Immatics N.V. (NASDAQ: IMTX, “Immatics” or the “Company”), a clinical-stage biopharmaceutical company and the global leader in precision targeting of PRAME, today announced the presentation of updated data from 16 patients with metastatic uveal melanoma treated with anzu-cel PRAME cell therapy.
The uveal melanoma data from the ongoing Phase 1b trial will be presented today at the European Society for Medical Oncology (ESMO) Congress 2025 during the Presidential Symposium III by Sapna Patel, M.D., Professor of Medicine, University of Colorado Cancer Center. The slides are accessible in the ‘Events & Presentations’ section of the Investors & Media section of the Company’s website.
“Patients with metastatic uveal melanoma face a poor prognosis and represent a population in need of better outcomes, given the limited options currently available,” said Sapna Patel, M.D. “I believe the results with anzu-cel presented today signal a much-needed breakthrough for patients with metastatic uveal melanoma. These findings highlight the potential of anzu-cel to redefine the treatment paradigm for uveal melanoma and bring new hope to patients who urgently need more effective options.”
“Our goal is to leverage every opportunity to bring innovative PRAME therapies to patients with limited treatment options,” said Cedrik Britten, M.D., Chief Medical Officer at Immatics. “The continued strong clinical data presented today reinforce our conviction in maximizing the potential of our PRAME cell therapy, anzu-cel, and expanding its development into metastatic uveal melanoma, a rare cancer with very high unmet medical need. We are excited to further execute on our PRAME franchise and bring meaningful progress to patients in need.”
Presidential Symposium III Presentation Summary – Anzu-cel Phase 1b Trial
Patient Population: Difficult-to-treat patient population with metastatic uveal melanoma
As of September 24, 2025, 16 patients with metastatic uveal melanoma were administered a one-time infusion of anzu-cel at the recommended Phase 2 dose (RP2D, 1 to 10 billion total TCR T cells) as part of the anzu-cel Phase 1b dose expansion. Patients received a median infused TCR T-cell dose of ~4 billion (range 1.62 - 8.43 billion TCR T cells) and had a median of 2 lines of prior systemic treatments. Patients had a median target lesion sum of a diameter of 103 mm (ranging from 31 to 210 mm), and
Anti-tumor Activity and Durability: Continued strong anti-tumor activity and durability of anzu-cel PRAME cell therapy
Updated data of a one-time infusion of anzu-cel PRAME cell therapy demonstrated promising benefit in a difficult-to-treat population with limited effective treatment options:
- Confirmed objective response rate (cORR) of
67% (10/151) - Disease control rate (DCR) of
88% (14/16) - Median duration of response (mDOR) of 11 months (min 4.4, max 31.6 months)
- Median progression-free survival (mPFS) of 8.5 months (min 1.4, max 32.9) at a mFU of 10.4 months. The PFS rate was
69% at six months and39% at 12 months - Median overall survival (mOS) not reached (min 4.3+, max 34.2+ months) at a mFU of 14.3 months. The OS rate was
71% at 12 months
Anti-tumor activity was observed in liver and extrahepatic metastases, including lung, lymph node, abdomen/peritoneum and others. 14/16 patients had target lesions in the liver and treatment with anzu-cel led to a median shrinkage in liver target lesion size of
Notably, 11 out of the 16 patients received a TCR bispecific (ten gp-100-targeting, one PRAME-targeting) as prior systemic treatment line, and thereof, six achieved a confirmed partial response, one a partial response and three stable disease. These results demonstrate anti-tumor activity of anzu-cel in patients who received prior TCR-based therapies.
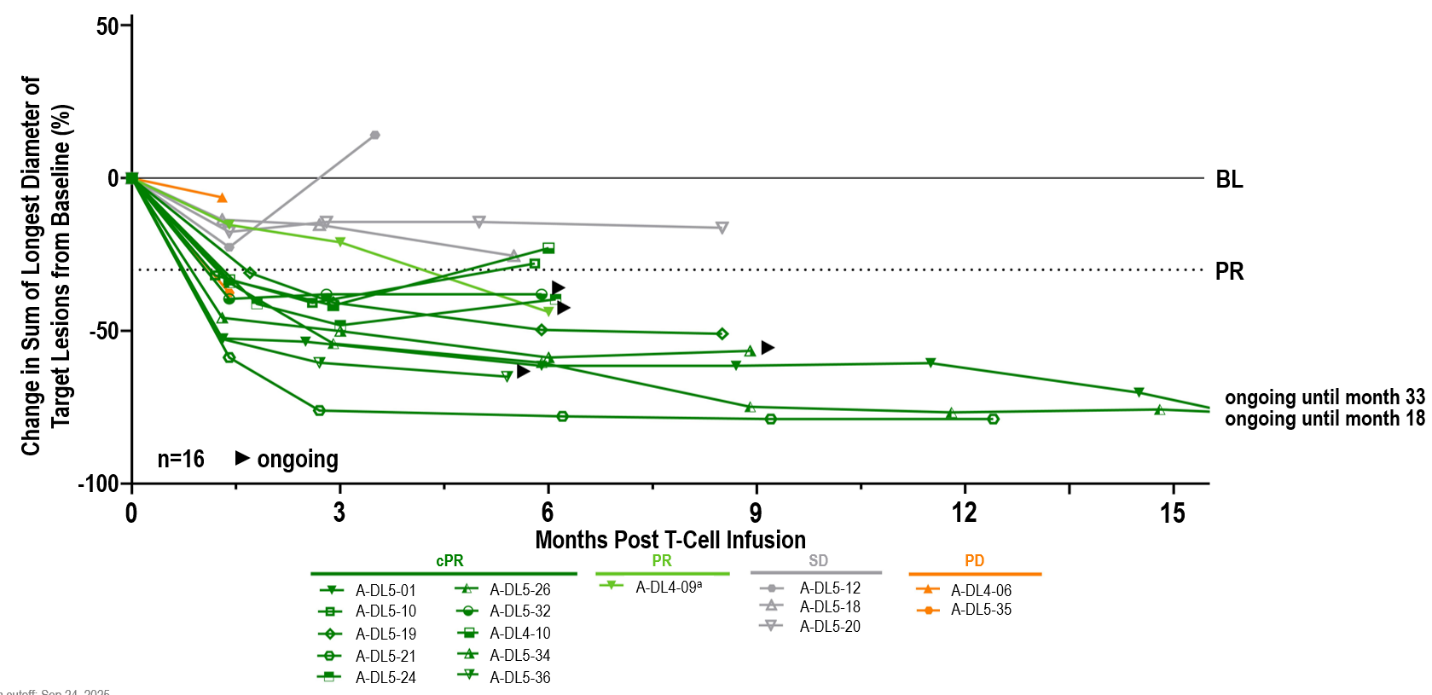
aPatient out of study at data cutoff (withdrew consent); BL, baseline; (c)PR, (confirmed) partial response; PD, progressive disease; SD, stable disease.
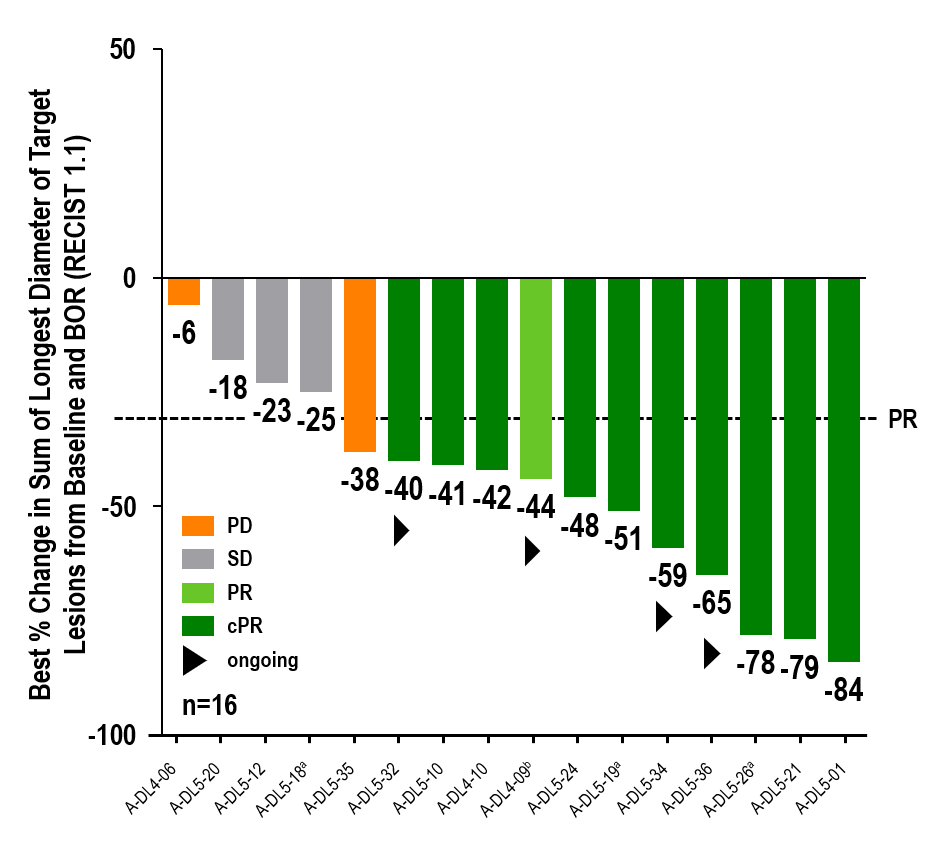
aMaximum change of target lesions and RECIST1.1 response at different timepoints. bPatient off study at data cutoff date (withdrew consent). 14/16 patients had liver target lesions with median best change of longest diameter of liver target lesions (range) of -
Safety: Favorable tolerability in uveal melanoma, generally consistent with full anzu-cel tolerability profile
The most frequent treatment-emergent adverse events (TEAs) were anticipated cytopenias associated with lymphodepletion. Expected and manageable cytokine release syndrome (CRS) was mostly Grade 1 or 2, which is consistent with the mechanism of action (Grade 1:
Tolerability in the uveal melanoma subset was generally consistent with the full anzu-cel tolerability profile in the Phase 1b.
Development Path for Anzu-cel in Metastatic Uveal Melanoma
Based on the promising clinical data in patients with metastatic uveal melanoma, Immatics has initiated a Phase 2 cohort with approximately 30 uveal melanoma patients planned. The cohort is being conducted at select centers in the U.S. and Germany with deep expertise in uveal melanoma. Given the high prevalence of PRAME expression in uveal melanoma, prospective PRAME testing is no longer required for inclusion in the clinical trial.
The consistent tolerability, anti-tumor activity and pharmacokinetic profile of anzu-cel across both uveal and cutaneous melanoma provide a strong rationale for pursuing a parallel late-stage development strategy to serve both patient populations.
About PRAME
PRAME is a target expressed in more than 50 cancers. Immatics is the global leader in precision targeting of PRAME and has the broadest PRAME franchise with the most PRAME indications and modalities. The Immatics PRAME franchise currently includes three product candidates, two therapeutic modalities and a combination therapy that target PRAME: anzu-cel (IMA203) PRAME cell therapy, IMA203CD8 PRAME cell therapy (GEN2), IMA402 PRAME bispecific, anzu-cel in combination with Moderna’s PRAME cell therapy enhancer.
About Anzu-cel (IMA203) PRAME Cell Therapy
Anzu-cel (anzutresgene autoleucel; IMA203) is a PRAME-directed TCR T-cell therapy engineered to recognize an intracellular PRAME-derived peptide presented by HLA-A*02:01 on the cell surface and initiate a potent and specific anti-tumor response. Anzu-cel PRAME cell therapy is currently being evaluated in a registration-enabling randomized controlled Phase 3 trial, “SUPRAME,” in patients with unresectable or metastatic cutaneous melanoma who have disease progression on or after treatment with at least one checkpoint inhibitor. In parallel, the Phase 1b clinical trial in patients with PRAME cancers is ongoing with a focus on uveal melanoma.
About Immatics
Immatics is committed to making a meaningful impact on the lives of patients with cancer. We are the global leader in precision targeting of PRAME, a target expressed in more than 50 cancers. Our cutting-edge science and robust clinical pipeline form the broadest PRAME franchise with the most PRAME indications and modalities, spanning TCR T-cell therapies and TCR bispecifics.
Immatics intends to use its website www.immatics.com as a means of disclosing material non-public information. For regular updates, you can also follow us on LinkedIn and Instagram.
Forward-Looking Statements
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or the Company’s future financial or operating performance. For example, statements concerning timing of data read-outs for product candidates, the timing, outcome and design of clinical trials, the nature of clinical trials (including whether such clinical trials will be registration-enabling), the timing of IND, CTA or BLA filings, estimated market opportunities of product candidates, the Company’s focus on partnerships to advance its strategy, and other metrics are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “plan”, “target”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management's control including general economic conditions and other risks, uncertainties and factors set forth in the Company’s Annual Report on Form 20-F and other filings with the Securities and Exchange Commission (SEC). Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. The Company undertakes no duty to update these forward-looking statements. All the scientific and clinical data presented within this press release are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification.
For more information, please contact:
Media
Trophic Communications
Phone: +49 151 74416179
immatics@trophic.eu
Immatics N.V.
Jordan Silverstein
Head of Strategy
Phone: +1 346 319-3325
InvestorRelations@immatics.com
- END -
1Excluding one patient who withdrew consent with ongoing unconfirmed response.
Attachment








