Intensity Therapeutics, Inc. Announces Publication of Clinical Results of INT230-6 for the Treatment of Metastatic or Refractory Cancers in eBioMedicine, a Lancet Discovery Science Journal
Intensity Therapeutics (Nasdaq: INTS) announced publication in eBioMedicine of phase 1/2 results for intratumoral INT230-6 in metastatic or refractory solid tumors (Online First Oct 29, 2025).
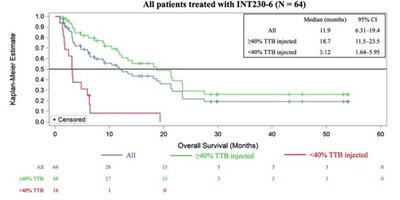
Key outcomes: disease control rate 75% (48/64), overall median survival 11.9 months, sarcoma subset mOS 21.3 months. In an exploratory analysis treating >40% total tumor burden, mOS was 18.7 months vs 3.1 months when <40% treated (HR 0.17; P<0.0001). Approximately 20% of >40% patients showed uninjected tumor shrinkage (abscopal effects). Safety: no dose‑limiting toxicities, grade 3 events in 10.9%, no grade 4/5 treatment‑related AEs. Pharmacokinetics: >95% of active agents remained in injected tumors. The company will host an author webinar on Oct 31, 2025 at 9:00 AM ET.
Intensity Therapeutics (Nasdaq: INTS) ha annunciato la pubblicazione su eBioMedicine dei risultati di fase 1/2 per INT230-6 somministrato intratumoralmente in tumori solidi metastatici o refrattari (Online First del 29 ottobre 2025).
Risultati chiave: tasso di controllo della malattia 75% (48/64), sopravvivenza globale mediana 11,9 mesi, mOS per sarcoma 21,3 mesi. In un’analisi esplorativa su un carico tumorale totale >40%, la mOS era 18,7 mesi vs 3,1 mesi quando >40% era trattato (HR 0,17; P<0,0001). Circa 20% dei pazienti con carico >40% hanno mostrato regressione dei tumori non iniettati (effetti abscopali). Sicurezza: nessuna tossicità dose-limitante, eventi di grado 3 in 10,9%, nessun evento avverso legato al trattamento di grado 4/5. Proprietà farmacocinetica: >95% dei farmaci attivi è rimasto nei tumori iniettati. L’azienda terrà un webinar per autori il 31 ottobre 2025 alle 9:00 ET.
Intensity Therapeutics (Nasdaq: INTS) anunció la publicación en eBioMedicine de los resultados de fase 1/2 de INT230-6 administrado intratumoralmente en tumores sólidos metastásicos o refractarios (Online First del 29 de octubre de 2025).
Resultados clave: tasa de control de la enfermedad 75% (48/64), supervivencia global mediana 11,9 meses, mOS de sarcoma 21,3 meses. En un análisis exploratorio tratando una carga tumoral total >40%, la mOS fue 18,7 meses frente a 3,1 meses cuando se trató <40% (HR 0,17; P<0,0001). Aproximadamente 20% de los pacientes con >40% de carga mostraron encogimiento de tumores no inyectados (efectos abscópulos). Seguridad: sin toxicidades dosis-limitantes, eventos de grado 3 en 10,9%, sin AE relacionados con el tratamiento de grado 4/5. Farmacocinética: >95% de los agentes activos se mantuvieron en los tumores inyectados. La empresa organizará un seminario web con autores el 31 de octubre de 2025 a las 9:00 a.m. ET.
Intensity Therapeutics (나스닥: INTS)는 eBioMedicine에 전이성 또는 난치성 고형 종양에서의 종양내투여 INT230-6의 1/2상 결과를 Online First로 2025년 10월 29일 게재했다고 발표했습니다.
주요 결과: 질병 조절률 75% (48/64), 전체 중간 생존기간 11.9개월, 육종 하위집단의 mOS 21.3개월. 총 종양 부담이 >40%인 탐색적 분석에서 mOS는 18.7개월였고 <40% 미만 치료 시 3.1개월이었습니다 (HR 0.17; P<0.0001). 약 40% 이상인 환자에서 치료 주사 부위 외의 종양 감소(앱스코풀 효과)가 관찰되었습니다. 안전성: 용량 제한 독성 없음, 등급 3 사건 10.9%, 등급 4/5 치료 관련 AE 없음. 약동학: 활성 제제의 >95%가 주사된 종양에 남아 있었습니다. 회사는 2025년 10월 31일 오후 9:00(동부표준시)에 저자 웨비나를 개최할 예정입니다.
Intensity Therapeutics (Nasdaq: INTS) a annoncé la publication dans eBioMedicine des résultats de phase 1/2 pour INT230-6 administré intratumoralement dans des tumeurs solides metastatiques ou réfractaires (Online First du 29 octobre 2025).
Résultats clés : taux de contrôle de la maladie 75% (48/64), survie globale médiane 11,9 mois, mOS du sous-groupe sarcome 21,3 mois. Dans une analyse exploratoire traitant une charge tumorale totale >40%, le mOS était 18,7 mois contre 3,1 mois lorsque >40% était traité (HR 0,17; P<0,0001). Environ 20% des patients avec >40% de charge ont montré une réduction des tumeurs non injectées (effets abscopales). Sécurité : aucune toxicité limitante de dose, événements de grade 3 dans 10,9%, aucun événement lié au traitement de grade 4/5. Pharmacocinétique : >95% des agents actifs sont restés dans les tumeurs injectées. L'entreprise organisera un webinaire d'auteurs le 31 octobre 2025 à 9h00 ET.
Intensity Therapeutics (Nasdaq: INTS) kündigte die Veröffentlichung in eBioMedicine der Phase-1/2-Ergebnisse von INT230-6, das intratumoral verabreicht wird, bei metastasierenden oder refraktären soliden Tumoren an (Online First am 29. Oktober 2025).
Schlüsselergebnisse: Disease-Control-Rate 75% (48/64), medianes Gesamtüberleben 11,9 Monate, mOS der Sarcoma-Untergruppe 21,3 Monate. In einer explorativen Analyse bei einer Gesamt-Tumorlast >40% betrug das mOS 18,7 Monate vs 3,1 Monate bei >40% behandelten (HR 0,17; P<0,0001). Ungefähr 20% der Patienten mit >40% Last zeigten Regression un-injizierter Tumore (Abscopal-Effekte). Sicherheit: keine dosislimitierenden Toxizitäten, Grad-3-Ereignisse 10,9%, keine Grad-4/5 behandlungsbezogenen AE. Pharmakokinetik: >95% der aktiven Wirkstoffe verbleiben in den injizierten Tumoren. Das Unternehmen wird am 31. Oktober 2025 um 9:00 Uhr ET einen Autor*innen-Webinar veranstalten.
Intensity Therapeutics (ناسداك: INTS) أعلنت نشرة في eBioMedicine عن نتائج المرحلة 1/2 لاختبار INT230-6 بالحقن داخل الورم في أورام صلبة متقدمة أو مقاومة (Online First في 29 أكتوبر 2025).
النتائج الرئيسية: معدل التحكم بالمرض 75% (48/64)، البقاء على قيد الحياة الوسيط الكلي 11.9 شهرًا، البقاء الوسيط للملازمة ساركومة 21.3 شهرًا. في تحليل استكشافي يعالج عبء الورم الكلي >40%، كان mOS 18.7 شهرًا مقابل 3.1 شهرًا عندما يعالج <40% (HR 0.17; P<0.0001). نحو 20% من المرضى >40% أظهروا تقليص الورم غير المحقون (تأثيرات أبسكوبال). السلامة: لا سمية مرتبطة بالجرعة، أحداث من الدرجة 3 في 10.9%، لا أحداث مرتبطة بالعلاج من الدرجة 4/5. الكيمياء الدوائية: >95% من العوامل النشطة بقيت في الأورام المحقونة. ستستضيف الشركة ندوة عبر الإنترنت للمؤلفين في 31 أكتوبر 2025 الساعة 9:00 صباحًا بتوقيت شرق الولايات المتحدة.
- Disease control rate 75% (48/64)
- Median overall survival 11.9 months
- Sarcoma subset median overall survival 21.3 months
- mOS 18.7 months when >40% total tumor burden treated (HR 0.17; P<0.0001)
- Pharmacokinetics: >95% of active agents retained in injected tumors
- Phase 1/2 open‑label dose escalation design limits definitive efficacy conclusions
- Treatment‑related grade 3 adverse events in 10.9% of monotherapy patients
Insights
Phase 1/2 publication reports favorable safety and survival signals for intratumoral INT230-6, with a strong subgroup survival benefit.
INT230-6 showed a disease control rate of
The exploratory analysis that treated >40% of total tumor burden showed a disease control rate of
These data suggest a local delivery mechanism that achieves high intratumoral exposure and measurable systemic immune activation, evidenced by increased activated CD4+ and CD8+ T cells in matched biopsies and observed abscopal responses. Key dependencies include reproducibility of the >40% tumor burden dosing effect, confirmation of survival benefit in randomized cohorts, and safety at higher absolute volumes (up to
The paper features a comprehensive evaluation of data, including disease control rate, overall survival, immune activation, abscopal effects, tumor necrosis, dose ranging, and safety
The manuscript is open access
The Company will host a webinar with the paper's lead and senior authors from the University of
Jacob Stephen Thomas, M.D. Assistant Professor of Clinical Medicine at Keck School of Medicine of the University of
The manuscript includes the following data results:
- In heavily pretreated patients with advanced disease having over 20 different types of cancer who had progressed following multiple prior lines of therapy, intratumoral INT230-6 achieved:
- A disease control rate of
75% (48/64 patients) and median overall survival (mOS) of 11.9 months; these results compare favorably in phase 1/2 studies that historically reported an mOS of 4 to 7 months - In a metastatic sarcoma subset population receiving only INT230-6, the median overall survival was 21.3 months
- A disease control rate of
- In an exploratory analysis comparing patients receiving INT230-6 at a total dose (in mL) that treated greater than
40% of the patient's total tumour burden ("TTB") compared to those treated with less than40% of their TTB, the:- Disease control rate was
83.3% (40/48) compared to50% (8/16) - Median overall survival was 18.7 months (
95% CI: 11.5–23.5) compared to 3.1 months (95% CI: 1.6–5.9) with a hazard ratio (HR) of 0.17 (95% CI: 0.081–0.342); P<0.0001 (see Figure 1 below) - Improved survival was consistent across a range of low to high tumor burden and tumor sizes
- Disease control rate was
- Approximately
20% of patients in the >40% group had uninjected tumors shrink, abscopal effects - Fifteen of 64 patients survived for more than 21 months
- INT230-6 induced a qualitative decrease in proliferating cancer cells in injected tumors and a qualitative increase in activated T-cells infiltrating the tumor microenvironment
- No dose-limiting toxicities were reported among 64 monotherapy patients; seven patients had a grade 3 (
10.9% ) with no grade 4 or 5 treatment-related adverse events - Pharmacokinetic results showed that greater than
95% of the active cytotoxic agents remained in the injected tumors
"INT230-6 is a local treatment that kills cancer using a diffusion process following direct injection into tumors. The trial demonstrated favorable safety and promising efficacy in patients with advanced metastatic cancers who had failed a median of three prior lines of therapy. The disease control rates and median survival compare favorably to those historically seen for such a diverse set of refractory cancer types in a phase 1/2 study," said Jacob S. Thomas, M.D. "There were also several learnings about INT230-6 dosing and safety gained during this trial. The pharmacokinetic data indicated that high rates of the drug are absorbed by the injected tumor, with minimal leakage, even at doses as high as 175 mL administered to a single tumor. These results are consistent with the low incidence of grade 3 adverse events observed."
"The mechanism by which cancer is killed through the diffusion of cytotoxic agents following intratumoral injection of INT230-6 and systemic immune activation, as observed in preclinical models, translated well in the human setting. Uninjected tumors shrinking from a locally administered therapy, referred to as abscopal effects, are generally rare for local therapies. Yet, an abscopal effect was observed in at least
"This comprehensive paper is the culmination of over a decade of nonclinical and clinical research. The article describes the development of a new technology to destroy tumors using molecular agents that can disperse potent anti-cancer compounds within injected tumors and deliver them into cancer cells. We believe these are the first clinical results where a locally administered therapy used alone could potentially extend survival for patients with metastatic disease," said Lewis H. Bender, Founder, President, and CEO of Intensity Therapeutics, Inc. "As Drs. Thomas and El-Khoueiry noted, our paper reports that INT230-6 injected into visible tumors in metastatic patients at an amount based on the size of the injected tumors supports the hypothesis that INT230-6 causes immunologic cancer cell death, even in cancers that are considered immunologically cold. Given the drug's mechanism of action and the data reported in this paper from over 20 types of metastatic solid cancers, such as breast, sarcoma, pancreatic, lung, and head and neck, we believe the study results show the potential of INT230-6 to achieve clinical benefit for metastatic patients of multiple cancer types with or without the use of radiation, systemic drugs or immunotherapy. As a result, we have initiated randomized controlled studies, including a Phase 3 study in sarcoma (NCT06263231)."
The Company will be hosting a conference call featuring two key authors of the study on Friday, October 31, 2025 at 9:00AM ET to discuss the results. Interested parties can access the call by clicking here: https://event.choruscall.com/mediaframe/webcast.html?webcastid=6uG6ARFf. Participants are encouraged to log on at least 10 minutes prior to the start of the event.
About INT230-6
INT230-6, Intensity's lead proprietary investigational product candidate, is designed for direct intratumoral injection. INT230-6 was discovered using Intensity's proprietary DfuseRx℠ technology platform. The drug consists of two proven, potent anti-cancer agents, cisplatin and vinblastine sulfate, and a diffusion and cell penetration enhancer molecule ("SHAO") that non-covalently conjugates to the two payload drugs, facilitating the dispersion of potent cytotoxic drugs throughout tumors and allowing the active agents to diffuse into cancer cells. These agents remain in the tumor, resulting in a favorable safety profile. In addition to local disease control and direct tumor killing, INT230-6 causes a release of a bolus of neoantigens specific to the malignancy, leading to immune system engagement and systemic anti-tumor effects. Importantly, these effects are mediated without immunosuppression, which often occurs with systemic chemotherapy.
About Study Intensity's Clinical Study IT-01
IT-01 was Intensity's first-in-human, open-label, single-arm phase 1/2 study (NCT03058289) using INT230-6. The study was conducted in patients with advanced, refractory, or metastatic solid tumors at six clinical sites in addition to USC. Other investigators were from Johns Hopkins University, Princess Margaret Hospital in
About eBioMedicine
eBioMedicine is a leading open-access translational research journal within the Lancet Discovery Group of journals. eBioMedicine encompasses the spectrum of biomedical research, ranging from preclinical studies with clear human relevance to proof-of-concept, first-in-human studies, and early-phase clinical trials. The journal's editors are dedicated to publishing original research that investigates the basic determinants of human health and disease, the discovery and characterization of new therapeutic targets and treatments, and the identification of biomarkers and diagnostic tools that may help researchers and clinicians better understand and monitor disease. The Journal welcomes studies that elucidate, or aims to modify, disease pathways and mechanisms—to advance knowledge in any biomedical discipline with relevance to human health. The Impact Factor of eBioMedicine is 10.8, according to The Lancet. This indicates that the journal's content is highly cited within the scientific community, particularly within the two years preceding the Journal Citation Reports year.
About Intensity
Intensity is a late-stage clinical biotechnology company whose novel engineered chemistry enables aqueous cytotoxic-containing drug formulations to mix and saturate a tumor's dense, high-fat, pressurized environment following direct intratumoral injection. As a result of the saturation, Intensity's clinical trials have demonstrated the ability of INT230-6 to kill tumors and elicit an adaptive immune response within days of injection, representing a new approach to cancer cell death that holds the potential to shift the treatment paradigm and turn many deadly cancers into chronic diseases even for malignancies that do not respond to conventional immunotherapy. Intensity has completed two clinical studies that enrolled over 200 patients using INT230-6: a Phase 1/2 dose escalation study in metastatic cancers including sarcomas (NCT03058289), and a Phase 2 randomized control clinical trial in locally advanced breast cancer (the "INVINCIBLE-2 Study") (NCT04781725) in women without undergoing chemotherapy prior to their surgery. The Company initiated a Phase 3 trial in soft tissue sarcoma (the "INVINCIBLE-3 Study") (NCT06263231), testing INT230-6 as second or third-line monotherapy compared to the standard of care ("SOC") with overall survival as an endpoint. Intensity also initiated a Phase 2 study in collaboration with The Swiss Group for Clinical Cancer Research, formerly SAKK, now the Swiss Cancer Institute (the "INVINCIBLE-4 Study") (NCT06358573) as part of a Phase 2/3 program evaluating INT230-6 followed by the SOC immunochemotherapy and the SOC alone for patients with presurgical triple-negative breast cancer. Pathological complete response ("pCR") is the endpoint. For more information about Intensity, including publications, papers, and posters about its novel approach to cancer therapeutics, visit www.intensitytherapeutics.com or review our SEC filings.
Forward-Looking Statements
Certain statements in this press release may constitute "forward-looking statements" within the meaning of the United States Private Securities Litigation Reform Act of 1995, as amended to date. These statements include, but are not limited to, statements relating to the Company's expected future plans, cash runway, development activities, projected milestones, business activities or results. When or if used in this communication, the words "may," "could," "should," "anticipate," "believe," "estimate," "expect," "intend," "plan," "predict" and similar expressions and their variants, as they relate to the Company or its management, may identify forward-looking statements. The forward-looking statements contained in this press release are based on management's current expectations and projections about future events. Nevertheless, actual results or events could differ materially from the plans, intentions, and expectations disclosed in, or implied by, the forward-looking statements. These risks and uncertainties, many of which are beyond our control, include: the initiation, timing, progress and results of future preclinical studies and clinical trials and research and development programs; the need to raise additional funding before the Company can expect to generate any revenues from product sales; plans to develop and commercialize product candidates; the timing or likelihood of regulatory filings and approvals; the ability of the Company's research to generate and advance additional product candidates; the risk that product candidates that appear promising in early research and clinical trials do not demonstrate safety and/or efficacy in larger-scale or later clinical trials; the implementation of the Company's business model, strategic plans for the Company's business, product candidates and technology; commercialization, marketing and manufacturing capabilities and strategy; the rate and degree of market acceptance and clinical utility of the Company's system; the Company's competitive position; the Company's intellectual property position; developments and projections relating to the Company's competitors and its industry; the Company's ability to maintain and establish collaborations or obtain additional funding; expectations related to the use of cash and cash equivalents and investments; our potential inability to satisfy the Nasdaq Capital Market's requirements for continued listing and be subject to delisting; estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and other risks described in the section entitled "Risk Factors" in the Company's Annual Report on Form 10-K for the year ended December 31, 2024 and in the Company's subsequent SEC filings, which can be obtained on the SEC website at www.sec.gov. Readers are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date on which they are made and reflect management's current estimates, projections, expectations and beliefs. The Company does not plan to update any such forward-looking statements and expressly disclaims any duty to update the information contained in this press release except as required by law.
Investor Relations Contact:
Justin Kulik
Justin@coreir.com
CORE IR
(516) 222-2560
Media Contact:
Matt Cossel
CORE IR
pr@coreir.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/intensity-therapeutics-inc-announces-publication-of-clinical-results-of-int230-6-for-the-treatment-of-metastatic-or-refractory-cancers-in-ebiomedicine-a-lancet-discovery-science-journal-302599519.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/intensity-therapeutics-inc-announces-publication-of-clinical-results-of-int230-6-for-the-treatment-of-metastatic-or-refractory-cancers-in-ebiomedicine-a-lancet-discovery-science-journal-302599519.html
SOURCE Intensity Therapeutics Inc.