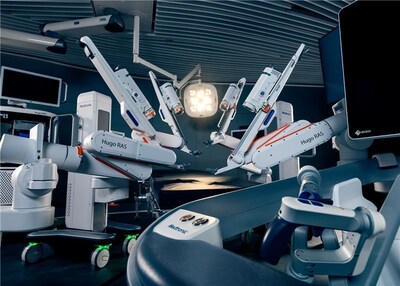
Medtronic announces FDA clearance of Hugo™ robotic-assisted surgery system for urologic surgical procedures
Rhea-AI Summary
Medtronic (NYSE: MDT) announced FDA clearance of the Hugo™ robotic-assisted surgery (RAS) system for use in minimally invasive urologic procedures including prostatectomy, nephrectomy, and cystectomy.
The release notes the Expand URO IDE study—described as the largest US multi-port robotic urology trial—met primary safety and effectiveness endpoints, and estimates those urologic procedures total about 230,000 surgeries per year in the U.S. Medtronic plans a purposeful U.S. launch with broader specialty indications expected later.
Positive
- FDA clearance for Hugo RAS in urologic procedures (Dec 3, 2025)
- Expand URO study met primary safety and effectiveness endpoints
- 230,000 U.S. urologic surgeries per year covered by indications
- Established use in tens of thousands of procedures across >30 countries
Negative
- Initial U.S. clearance limited to urologic procedures only
- Rollout described as a purposeful launch, implying phased availability
Insights
FDA clearance of the Hugo™ RAS system expands U.S. robotic options for urologic surgery and follows a successful pivotal study.
The Hugo RAS system adds a modular, multi‑arm robotic platform to U.S. urologic practice, enabling minimally invasive prostatectomy, nephrectomy, and cystectomy and integrating with the Touch Surgery digital ecosystem for training, tele‑proctoring and post‑op case review. The Expand URO IDE study met primary safety and effectiveness endpoints, and the system has prior international use in tens of thousands of procedures across more than 30 countries.
Key dependencies and risks include hospital adoption decisions, operational deployment across care settings, and the pace of additional specialty indications; these factors will shape utilization despite clearance. Watch initial U.S. launch partnerships, published procedural outcomes from early U.S. sites, and timing for follow‑on indications for general and gynecologic surgery over the next 12–36 months, with early utilization signals likely within the first year after launch.
Hugo RAS system brings choice to the
GALWAY,
The
"This is an incredibly exciting day for healthcare in
Thoughtfully designed with input from surgeons and hospital administrators to shape the future of surgery, the Hugo RAS system includes three main differentiators:
- Modular Design: The Hugo RAS system's innovative modular design means the robotic arms can be easily moved, shared, and deployed across any care setting — helping to maximize utilization and providing surgeons flexibility to customize their approach to optimize anatomical access for each patient's unique needs. The open design of the surgeon console provides greater situational awareness and visualization, reduces physical strain, and creates enhanced training opportunities for surgical teams — enabling better bedside communication and team integration.
- Digital Ecosystem: The Hugo RAS system connects seamlessly with the Touch Surgery™ ecosystem, which provides pre-operative training tools, remote tele-proctoring capabilities, and AI-powered post-operative case insights.† Surgeons may securely access case videos minutes after a procedure is completed, supporting continuous improvement and collaboration among hospital teams and with their peers in the global surgical community.
- Differentiated Partnership: Medtronic is the first and only company that can meet surgeon needs across all surgical modalities — open, laparoscopic, and robotic-assisted. The addition of the Hugo RAS system provides surgical teams access to world-class robotic training, deep clinical and technical expertise, and choice when determining the best surgical approach for each patient's unique needs, with the opportunity to use trusted Medtronic technologies and instruments as technology advances.
"The Hugo RAS system represents a new and exciting approach to robotic-assisted surgery," said Dr. James Porter, a urologic surgeon and chief medical officer for Robotic Surgical Technologies and Digital Technologies within the Surgical business at Medtronic. "We're excited for surgical teams in the
With FDA clearance, the Hugo RAS system is indicated for use in minimally invasive urologic surgical procedures including prostatectomy, nephrectomy, and cystectomy — common procedures that account for about 230,000 surgeries per year in the
The Expand URO investigational device exemption clinical study — the largest ever completed for multi-port robotic-assisted urological surgery in the
Outside the
The introduction of the Hugo RAS system in the
For more information, visit medtronic.com/hugoishere.
†The Medtronic Touch Surgery™ ecosystem is not intended to direct surgery, or aid in diagnosis or treatment of a disease or condition. Performance Insights are available for select procedures, instruments, and anatomy.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Galway,
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Contacts:
Gary Jeanfaivre
Public Relations
+1-203-556-0777
Ryan Weispfenning
Investor Relations
+1-612-839-4549
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-announces-fda-clearance-of-hugo-robotic-assisted-surgery-system-for-urologic-surgical-procedures-302632328.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-announces-fda-clearance-of-hugo-robotic-assisted-surgery-system-for-urologic-surgical-procedures-302632328.html
SOURCE Medtronic plc